Chemistry:Dithiazanine iodide
From HandWiki
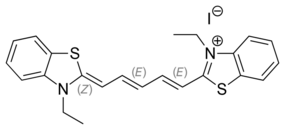
| |
| Names | |
|---|---|
| IUPAC name
3-Ethyl-2-[5-(3-ethyl-2-benzothiazolinylidene)-1,3-pentadienyl]-benzothiazolium iodide
| |
| Systematic IUPAC name
3-Ethyl-2-[(1E,3E,5Z)-5-(3-ethyl-1,3-benzothiazol-2(3H)-ylidene)penta-1,3-dien-1-yl]-1,3-benzothiazol-3-ium iodide | |
| Identifiers | |
3D model (JSmol)
|
|
| 3838938 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| UN number | 2811 |
| |
| Properties | |
| C23H23IN2S2 | |
| Molar mass | 518.48 g·mol−1 |
| Appearance | Green crystals |
| Melting point | Decomposes at 478.4 °F (248.0 °C) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H300, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Dithiazanine iodide is a chemical compound belonging to the group of polymethine dyes.[1] It is used as a veterinary anthelmintic for dogs.[3] It is a highly toxic chemical, with a lethal dose for humans of about 4–16 mg/kg by oral ingestion. The mechanism of toxicity is not well known but it is believed that this chemical interferes with cells' absorption of glucose, which is essential to obtain energy through cell respiration. [citation needed]
References
- ↑ 1.0 1.1 Cameo Chemicals. "Chemical data". NOAA. http://cameochemicals.noaa.gov/chemical/4976.
- ↑ 2.0 2.1 "Chemspider data". Chemspider. http://www.chemspider.com/Chemical-Structure.4642986.html.
- ↑ "Law about use". Justia. http://law.justia.com/cfr/title21/21-6.0.1.1.9.0.1.90.html.
 |

