Chemistry:Baloxavir marboxil
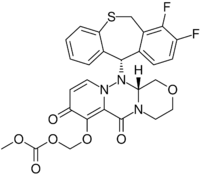 | |
| Clinical data | |
|---|---|
| Trade names | Xofluza |
| Other names | BXM (S-033188), BXA (S-033447) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618062 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C27H23F2N3O7S |
| Molar mass | 571.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Baloxavir marboxil, sold under the brand name Xofluza, is an antiviral medication for treatment of influenza A and influenza B.[4] It was approved for medical use both in Japan and in the United States in 2018,[7][8][9] and is taken as a single dose by mouth.[4] It may reduce the duration of flu symptoms by about a day, but is prone to selection of resistant mutants that render it ineffectual.[10]
Baloxavir marboxil was developed as a prodrug strategy, with its metabolism releasing the active agent, baloxavir acid (BXA). Baloxavir acid then functions as enzyme inhibitor, targeting the influenza virus' cap-dependent endonuclease activity, used in "cap snatching" by the virus' polymerase complex, a process essential to its life-cycle.[11]
The most common side effects of baloxavir marboxil include diarrhea, bronchitis, nausea, sinusitis, and headache.[12]
The US Food and Drug Administration (FDA) considers baloxavir marboxil to be a first-in-class medication.[13]
Medical uses
Baloxavir marboxil is an influenza medication, an antiviral,[14][4] for individuals who are twelve years of age or older,[5][14] that have presented symptoms of this infection for no more than 48 hours.[5][14][15] The efficacy of baloxavir marboxil administered after 48 hours has not been tested.[14]
In October 2019, the FDA approved an updated indication for the treatment of acute, uncomplicated influenza in people twelve years of age and older at risk of influenza complications.[4][9]
In November 2020, the FDA approved an updated indication to include post-exposure prevention of influenza (flu) for people twelve years of age and older after contact with an individual who has the flu.[4][12]
In August 2022, the FDA approved an updated indication to include post-exposure prevention of influenza (flu) for people five years of age and older after contact with an individual who has the flu.[4][16]
In the EU, baloxavir marboxil is indicated for the treatment of uncomplicated influenza and for post-exposure prophylaxis of influenza in individuals aged twelve years of age and older.[5]
Available forms
Baloxavir marboxil is available in tablet form and as granules for mixing in water.[12]
Resistance
In 2.2% of baloxavir recipients in the Phase II trial and in about 10% of baloxavir recipients in the Phase III trial, the infecting influenza strain had acquired resistance to the drug, due to variants of the polymerase protein displaying substitutions of isoleucine-38, specifically, the I38T, I38M, or I38F mutations.[17] There is continuing research into and clinical concern over the resistance appearing in recipients, in response to treatment with this drug.[18][19][20]
Contraindications
Baloxavir marboxil should not be co-administered with dairy products, calcium-fortified beverages, or laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminum or zinc.[12]
Side effects
Common side effects following the single dose administration of baloxavir marboxil include diarrhea, bronchitis, common cold, headache, and nausea.[4][15][7] Adverse events were reported in 21% of people who received baloxavir, 25% of those receiving placebo, and 25% of oseltamivir.[17]
Mechanism of action
Baloxavir marboxil is an influenza therapeutic agent, specifically, an enzyme inhibitor targeting the influenza virus' cap-dependent endonuclease activity, one of the activities of the virus polymerase complex.[21] In particular, it inhibits a process known as cap snatching, by which the virus derives short, capped primers from host cell RNA transcripts, which it then uses for polymerase-catalyzed synthesis of its needed viral mRNAs.[22] A polymerase subunit binds to the host pre-mRNAs at their 5' caps, then the polymerase's endonuclease activity catalyzes its cleavage "after 10–13 nucleotides". As such, its mechanism is distinct from neuraminidase inhibitors such as oseltamivir and zanamivir.[22]
Chemistry
Baloxavir marboxil is a substituted pyridone derivative of a polycyclic family, whose chemical synthesis has been reported in a number of ways by the company discovering it, Shionogi and Co. of Japan (as well as others); the Shionogi reports have appeared several times in the Japanese patent literature between 2016 and 2019, providing insight into possible industrial synthetic routes that may be in use.[23][24][25][26][27]
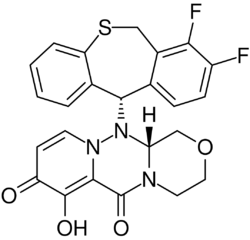
Baloxavir marboxil (BXM) is a prodrug whose active agent, baloxavir acid (BXA) is released rapidly in vivo, as the hydrolysis of baloxavir marboxil is catalyzed by arylacetamide deacetylases in cells of the blood, liver, and lumen of the small intestine.[29][30][31] The compound numbers for baloxavir marboxil and baloxavir acid used in publications by Shionogi and others during discovery and development (prior to assignment of a United States Adopted Name (USAN)) were, respectively, S-033188 and S-033447.[32] As reported in a review of the patent literature, the carbonic acid ester (carbonate) moiety of the prodrug—shown in the lower left-hand corner of the image above—was prepared during discovery and development research from a late stage 2-hydroxy-4-pyridone precursor by treatment with chloromethyl methyl carbonate.[29][33]
History
As of September 2018, in the only report of a Phase III randomized, controlled trial, baloxavir reduced the duration of influenza symptoms of otherwise healthy participants by about one day compared with a placebo treatment group, and comparable with what was seen for an oseltamivir treatment group.[15] On the first day after baloxavir was started in its treatment group of participants, viral loads decreased more than in participants in either the oseltamivir or placebo groups; however, after five days, the effect on viral load of the single dose of baloxavir was indistinguishable from the effect observed following the complete, 5-day regimen of oseltamivir in its treatment group.[17][verification needed]
Baloxavir marboxil was developed for the market by Shionogi Co., a Japan ese pharmaceutical company, and Switzerland -based Roche AG.[34] The names under which baloxavir marboxil and baloxavir acid appear in Shionogi research reporting are S-033188 and S-033447, respectively.[citation needed]
Society and culture
Legal status
Japan's Ministry of Health, Labour and Welfare (JMHLW) and the US Food and Drug Administration (FDA) approved baloxavir marboxil based on evidence of its benefits and side effects from two clinical trials in adult and pediatric participants with uncomplicated influenza (Trial 1, 1518T0821 and Trial 2, NCT02954354[35]),[4] involving 1119 participants.[15][36][better source needed][verification needed] Both trials included clinical sites and participants in Japan, with Trial 2 adding clinical locations in the United States.[4][15]
Baloxavir marboxil was approved for sale in Japan in February 2018.[37][better source needed] In October 2018, the FDA approved it for the treatment of acute uncomplicated influenza in people twelve years of age and older who have been symptomatic for no more than 48 hours.[7][8] The FDA application of baloxavir marboxil was granted priority review in the United States, and approval of Xofluza was granted to Shionogi & Co., Ltd. in October 2018.[7][8] Specifically, the FDA approved the use of baloxavir marboxil for people at high risk of developing influenza-related complications.[38] In October 2019, the FDA approved an updated indication for the treatment of acute, uncomplicated influenza in people twelve years of age and older at risk of influenza complications.[9] In November 2020, the FDA approved an updated indication to include post-exposure prevention of influenza (flu) for people twelve years of age and older after contact with an individual who has the flu.[12]
Baloxavir marboxil was approved for medical use in Australia in February 2020.[1]
The safety and efficacy of baloxavir marboxil, an antiviral drug taken as a single oral dose, was demonstrated in two randomized controlled clinical trials of 1,832 subjects where participants were assigned to receive either baloxavir marboxil, a placebo, or another antiviral flu treatment within 48 hours of experiencing flu symptoms.[7] In both trials, subjects treated with baloxavir marboxil had a shorter time to alleviation of symptoms compared with subjects who took the placebo.[7] In the second trial, there was no difference in the time to alleviation of symptoms between subjects who received baloxavir marboxil and those who received the other flu treatment.[7]
The safety and efficacy of baloxavir marboxil for post-flu exposure prevention is supported by one randomized, double-blind, controlled trial in which 607 subjects, twelve years of age and older who were exposed to a person with influenza in their household, received either a single dose of baloxavir marboxil or a single dose of a placebo.[12] Of these 607 subjects, 303 received baloxavir marboxil and 304 received the placebo.[12] The trial's primary endpoint was the proportion of subjects who were infected with influenza virus and presented with fever and at least one respiratory symptom from day 1 to day 10.[12] Of those who received baloxavir marboxil, 1% of subjects met these criteria, compared to 13% of subjects who received a placebo for the clinical trial.[12]
Baloxavir marboxil was approved for medical use in the European Union in January 2021.[5]
References
- ↑ 1.0 1.1 1.2 "Xofluza Australian prescription medicine decision summary". 2 March 2020. https://www.tga.gov.au/apm-summary/xofluza.
- ↑ "Xofluza Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=98624.
- ↑ "Summary Basis of Decision (SBD) for Xofluza". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00475&lang=en.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 "Xofluza- baloxavir marboxil tablet, film coated". 28 October 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e49e1a61-1b7c-4be5-ac84-af6240b511e7.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Xofluza EPAR". 9 November 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/xofluza. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Xofluza Product information". https://ec.europa.eu/health/documents/community-register/html/h1500.htm.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 "FDA approves new drug to treat influenza" (Press release). U.S. Food and Drug Administration (FDA). 24 October 2018. Archived from the original on 24 October 2019. Retrieved 23 October 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 8.0 8.1 8.2 "Drug Approval Package: Xofluza Film-Coated Tablets (Baloxavir marboxil)". U.S. Food and Drug Administration (FDA). 7 December 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210854Orig1s000TOC.cfm.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 9.0 9.1 9.2 "FDA Approval Package for 210854Orig1s001". U.S. Food and Drug Administration (FDA). 16 October 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/210854Orig1s001.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Baloxavir Marboxil for Uncomplicated Influenza: Mechanism of Action & Side Effects". https://en.huatengsci.com/article/106.html.
- ↑ "In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit". Antiviral Res. 160: 109–117. December 2018. doi:10.1016/j.antiviral.2018.10.008. PMID 30316915.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 "FDA Expands Approval of Influenza Treatment to Post-Exposure Prevention" (Press release). U.S. Food and Drug Administration (FDA). 23 November 2020. Archived from the original on 23 November 2020. Retrieved 23 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ (PDF) New Drug Therapy Approvals 2018 (Report). U.S. Food and Drug Administration (FDA). January 2019. https://www.fda.gov/media/120357/download. Retrieved 16 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 14.0 14.1 14.2 14.3 "Baloxavir Marboxil Monograph for Professionals". American Society of Health-System Pharmacists. 19 August 2019. https://www.drugs.com/monograph/baloxavir-marboxil.html.
- ↑ 15.0 15.1 15.2 15.3 15.4 "Drug Trial Snapshot: Xofluza". U.S. Food and Drug Administration (FDA). 23 July 2019. http://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-xofluza.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Genentech Announces FDA Approval of Xofluza to Treat Influenza in Children Aged Five and Older" (Press release). Genentech. 11 August 2022. Archived from the original on 12 August 2022. Retrieved 11 August 2022 – via Business Wire.
- ↑ 17.0 17.1 17.2 "Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents". N. Engl. J. Med. 379 (10): 913–923. September 2018. doi:10.1056/NEJMoa1716197. PMID 30184455.
- ↑ "Roche's flu med Xofluza drives drug resistance and may be a bad choice for kids, study says". 26 November 2019. https://www.fiercepharma.com/pharma/roche-s-flu-med-xofluza-drives-drug-resistance-and-may-be-a-bad-choice-for-kids-study-says.
- ↑ "New flu drug drives drug resistance in influenza viruses". EurekAlert (Press release). Archived from the original on 16 January 2021. Retrieved 8 January 2020.
- ↑ "Influenza A variants with reduced susceptibility to baloxavir isolated from Japanese patients are fit and transmit through respiratory droplets". Nature Microbiology 5 (1): 27–33. January 2020. doi:10.1038/s41564-019-0609-0. PMID 31768027.
- ↑ "At the centre: influenza A virus ribonucleoproteins". Nature Reviews. Microbiology 13 (1): 28–41. January 2015. doi:10.1038/nrmicro3367. PMID 25417656.
- ↑ 22.0 22.1 "Baloxavir marboxil: the new influenza drug on the market". Current Opinion in Virology 35: 14–18. April 2019. doi:10.1016/j.coviro.2019.01.006. PMID 30852344.
- ↑ Okamoto K, Ueno T, Hato Y, Hakogi T, Majima S, "Method for stereoselective preparation of substituted polycyclic pyridone derivative.", WO patent 2019070059, published 2019, assigned to Shionogi & Co., Ltd., Japan.
- ↑ Kawai M, Tomita K, Akiyama T, Okano A, Miyagawa M, "Pharmaceutical composition containing polycyclic pyridone derivatives as cap-dependent endonuclease (CEN) inhibitors.", JPWO patent 2018030463, published 2017, assigned to Shionogi and Co., Ltd., Japan.
- ↑ Shibahara S, Fukui N, Maki T, "Polycyclic pyridone derivative, crystal and preparation method thereof.", JPWO patent 2017221869, published 2017, assigned to Shionogi and Co., Ltd., Japan.
- ↑ Kawai M, Tomita K, Akiyama T, Okano A, Miyagawa M, "Preparation of polycyclic pyridone derivatives as cap-dependent endonuclease (CEN) inhibitors and prodrugs thereof.", JPWO patent 2016175224, published 2016, assigned to Shionogi and Co., Ltd., Japan
- ↑ Zhu X, Jiang W, "Process for preparation of substituted polycyclic carbamoylpyridone derivative and its prodrug.", CN patent 108440564, published 2018
- ↑ "Baloxavir". PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/124081876.
- ↑ 29.0 29.1 "Review of the Patent Literature: Synthesis and Final Forms of Antiviral Drugs Tecovirimat and Baloxavir Marboxil". Organic Process Research & Development 23 (7): 1298–1307. June 2019. doi:10.1021/acs.oprd.9b00144.
- ↑ "Molecular Dynamics Simulation reveals the mechanism by which the Influenza Cap-dependent Endonuclease acquires resistance against Baloxavir marboxil". Scientific Reports 9 (1): 17464. November 2019. doi:10.1038/s41598-019-53945-1. PMID 31767949. Bibcode: 2019NatSR...917464Y.
- ↑ "Evaluation of Drug-Drug Interaction Potential between Baloxavir Marboxil and Oseltamivir in Healthy Subjects". Clinical Drug Investigation 38 (11): 1053–1060. November 2018. doi:10.1007/s40261-018-0697-2. PMID 30203386.
- ↑ "Investigational antiviral therapies for the treatment of influenza". Expert Opinion on Investigational Drugs 28 (5): 481–488. May 2019. doi:10.1080/13543784.2019.1606210. PMID 31018720.
- ↑ "Baloxavir Marboxil". https://pharmaceutical-substances.thieme.com/ps/search-results?docUri=KD-02-0182.
- ↑ "A flu drug — shown to reduce the duration of symptoms — could upend treatment in U.S.". 27 June 2018. https://www.statnews.com/2018/06/27/flu-drug-single-pill/.
- ↑ A Study of S-033188 (Baloxavir Marboxil) Compared With Placebo or Oseltamivir in Otherwise Healthy Patients With Influenza. 3 November 2016. https://clinicaltrials.gov/ct2/show/NCT02954354. Retrieved 11 August 2022.
- ↑ "One-dose flu drug Xofluza gets nod from health ministry". Japan Times Online. 24 February 2018. https://www.japantimes.co.jp/news/2018/02/24/national/science-health/one-dose-flu-drug-xofluza-gets-nod-health-ministry/.
- ↑ "Xofluza (Baloxavir Marboxil) Tablets 10mg/20mg Approved For The Treatment Of Influenza Types A And B In Japan" (Press release). Shionogi & Co., Ltd. 23 February 2018. Archived from the original on 20 January 2021. Retrieved 23 February 2018 – via publicnow.com.
- ↑ "Genentech Announces FDA Approval of Xofluza (Baloxavir Marboxil) for People at High Risk of Developing Influenza-Related Complications" (Press release). Genentech. 17 October 2019. Archived from the original on 24 October 2019. Retrieved 23 October 2019 – via Business Wire.
Further reading
- "Baloxavir Marboxil for Prophylaxis against Influenza in Household Contacts". N. Engl. J. Med. 383 (4): 309–320. July 2020. doi:10.1056/NEJMoa1915341. PMID 32640124.
- "Baloxavir: game-changer or much ado about nothing?". Lancet Respir Med 6 (12): 903–904. December 2018. doi:10.1016/S2213-2600(18)30469-7. PMID 30420246.
External links
- "Baloxavir marboxil". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/baloxavir%20marboxil.
- "Baloxavir marboxil". 29 June 2018. https://www.sps.nhs.uk/medicines/baloxavir-marboxil/.
 |

