Chemistry:Methane clathrate
Methane clathrate (CH4·5.75H2O) or (8CH4·46H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice.[1][2][3][4][5][6] Originally thought to occur only in the outer regions of the Solar System, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediments on the ocean floors of the Earth.[7] Methane hydrate is formed when hydrogen-bonded water and methane gas come into contact at high pressures and low temperatures in oceans.
Methane clathrates are common constituents of the shallow marine geosphere and they occur in deep sedimentary structures and form outcrops on the ocean floor. Methane hydrates are believed to form by the precipitation or crystallisation of methane migrating from deep along geological faults. Precipitation occurs when the methane comes in contact with water within the sea bed subject to temperature and pressure. In 2008, research on Antarctic Vostok Station and EPICA Dome C ice cores revealed that methane clathrates were also present in deep Antarctic ice cores and record a history of atmospheric methane concentrations, dating to 800,000 years ago.[8] The ice-core methane clathrate record is a primary source of data for global warming research, along with oxygen and carbon dioxide.
Methane clathrates used to be considered as a potential source of abrupt climate change, following the clathrate gun hypothesis. In this scenario, heating causes catastrosphic melting and breakdown of primarily undersea hydrates, leading to a massive release of methane and accelerating warming. Current research shows that hydrates react very slowly to warming, and that it's very difficult for methane to reach the atmosphere after dissociation.[9][10] Some active seeps instead act as a minor carbon sink, because with the majority of methane dissolved underwater and encouraging methanotroph communities, the area around the seep also becomes more suitable for phytoplankton.[11] As the result, methane hydrates are no longer considered one of the tipping points in the climate system, and according to the IPCC Sixth Assessment Report, no "detectable" impact on the global temperatures will occur in this century through this mechanism.[12] Over several millennia, a more substantial 0.4–0.5 °C (0.72–0.90 °F) response may still be seen.[13]
General
Methane hydrates were discovered in Russia in the 1960s, and studies for extracting gas from it emerged at the beginning of the 21st century.[14]
Structure and composition
The nominal methane clathrate hydrate composition is (CH4)4(H2O)23, or 1 mole of methane for every 5.75 moles of water, corresponding to 13.4% methane by mass, although the actual composition is dependent on how many methane molecules fit into the various cage structures of the water lattice. The observed density is around 0.9 g/cm3, which means that methane hydrate will float to the surface of the sea or of a lake unless it is bound in place by being formed in or anchored to sediment.[15] One litre of fully saturated methane clathrate solid would therefore contain about 120 grams of methane (or around 169 litres of methane gas at 0 °C and 1 atm),[nb 1] or one cubic metre of methane clathrate releases about 160 cubic metres of gas.[14]
Methane forms a "structure-I" hydrate with two dodecahedral (12 vertices, thus 12 water molecules) and six tetradecahedral (14 water molecules) water cages per unit cell. (Because of sharing of water molecules between cages, there are only 46 water molecules per unit cell.) This compares with a hydration number of 20 for methane in aqueous solution.[16] A methane clathrate MAS NMR spectrum recorded at 275 K and 3.1 MPa shows a peak for each cage type and a separate peak for gas phase methane.[citation needed] In 2003, a clay-methane hydrate intercalate was synthesized in which a methane hydrate complex was introduced at the interlayer of a sodium-rich montmorillonite clay. The upper temperature stability of this phase is similar to that of structure-I hydrate.[17]

Natural deposits
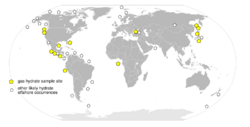
Methane clathrates are restricted to the shallow lithosphere (i.e. < 2,000 m depth). Furthermore, necessary conditions are found only in either continental sedimentary rocks in polar regions where average surface temperatures are less than 0 °C; or in oceanic sediment at water depths greater than 300 m where the bottom water temperature is around 2 °C. In addition, deep fresh water lakes may host gas hydrates as well, e.g. the fresh water Lake Baikal, Siberia.[18] Continental deposits have been located in Siberia and Alaska in sandstone and siltstone beds at less than 800 m depth. Oceanic deposits seem to be widespread in the continental shelf (see Fig.) and can occur within the sediments at depth or close to the sediment-water interface. They may cap even larger deposits of gaseous methane.[19]
Oceanic
Methane hydrate can occur in various forms like massive, dispersed within pore spaces, nodules, veins/fractures/faults, and layered horizons.[20] Generally, it is found unstable at standard pressure and temperature conditions, and 1 m3 of methane hydrate upon dissociation yields about 164 m3 of methane and 0.87 m3 of freshwater.[21][22][23] There are two distinct types of oceanic deposits. The most common is dominated (> 99%) by methane contained in a structure I clathrate and generally found at depth in the sediment. Here, the methane is isotopically light (δ13C < −60‰), which indicates that it is derived from the microbial reduction of CO2. The clathrates in these deep deposits are thought to have formed in situ from the microbially produced methane since the δ13C values of clathrate and surrounding dissolved methane are similar.[19] However, it is also thought that freshwater used in the pressurization of oil and gas wells in permafrost and along the continental shelves worldwide combines with natural methane to form clathrate at depth and pressure since methane hydrates are more stable in freshwater than in saltwater.[2] Local variations may be widespread since the act of forming hydrate, which extracts pure water from saline formation waters, can often lead to local and potentially significant increases in formation water salinity. Hydrates normally exclude the salt in the pore fluid from which it forms. Thus, they exhibit high electric resistivity like ice, and sediments containing hydrates have higher resistivity than sediments without gas hydrates (Judge [67]).[24]:9
These deposits are located within a mid-depth zone around 300–500 m thick in the sediments (the gas hydrate stability zone, or GHSZ) where they coexist with methane dissolved in the fresh, not salt, pore-waters. Above this zone methane is only present in its dissolved form at concentrations that decrease towards the sediment surface. Below it, methane is gaseous. At Blake Ridge on the Atlantic continental rise, the GHSZ started at 190 m depth and continued to 450 m, where it reached equilibrium with the gaseous phase. Measurements indicated that methane occupied 0-9% by volume in the GHSZ, and ~12% in the gaseous zone.[25][26]
In the less common second type found near the sediment surface, some samples have a higher proportion of longer-chain hydrocarbons (< 99% methane) contained in a structure II clathrate. Carbon from this type of clathrate is isotopically heavier (δ13C is −29 to −57 ‰) and is thought to have migrated upwards from deep sediments, where methane was formed by thermal decomposition of organic matter. Examples of this type of deposit have been found in the Gulf of Mexico and the Caspian Sea.[19]
Some deposits have characteristics intermediate between the microbially and thermally sourced types and are considered formed from a mixture of the two.
The methane in gas hydrates is dominantly generated by microbial consortia degrading organic matter in low oxygen environments, with the methane itself produced by methanogenic archaea. Organic matter in the uppermost few centimeters of sediments is first attacked by aerobic bacteria, generating CO2, which escapes from the sediments into the water column. Below this region of aerobic activity, anaerobic processes take over, including, successively with depth, the microbial reduction of nitrite/nitrate, metal oxides, and then sulfates are reduced to sulfides. Finally, methanogenesis becomes a dominant pathway for organic carbon remineralization.
If the sedimentation rate is low (about 1 cm/yr), the organic carbon content is low (about 1% ), and oxygen is abundant, aerobic bacteria can use up all the organic matter in the sediments faster than oxygen is depleted, so lower-energy electron acceptors are not used. But where sedimentation rates and the organic carbon content are high, which is typically the case on continental shelves and beneath western boundary current upwelling zones, the pore water in the sediments becomes anoxic at depths of only a few centimeters or less. In such organic-rich marine sediments, sulfate becomes the most important terminal electron acceptor due to its high concentration in seawater. However, it too is depleted by a depth of centimeters to meters. Below this, methane is produced. This production of methane is a rather complicated process, requiring a highly reducing environment (Eh −350 to −450 mV) and a pH between 6 and 8, as well as a complex syntrophic, consortia of different varieties of archaea and bacteria. However, it is only archaea that actually emit methane.
In some regions (e.g., Gulf of Mexico, Joetsu Basin) methane in clathrates may be at least partially derive from thermal degradation of organic matter (e.g. petroleum generation), with oil even forming an exotic component within the hydrate itself that can be recovered when the hydrate is disassociated.[27][28][citation needed] The methane in clathrates typically has a biogenic isotopic signature and highly variable δ13C (−40 to −100‰), with an approximate average of about −65‰ .[29][citation needed][30][31][32] Below the zone of solid clathrates, large volumes of methane may form bubbles of free gas in the sediments.[25][33][34]
The presence of clathrates at a given site can often be determined by observation of a "bottom simulating reflector" (BSR), which is a seismic reflection at the sediment to clathrate stability zone interface caused by the unequal densities of normal sediments and those laced with clathrates.
Gas hydrate pingos have been discovered in the Arctic oceans Barents sea. Methane is bubbling from these dome-like structures, with some of these gas flares extending close to the sea surface.[35]
Reservoir size
The size of the oceanic methane clathrate reservoir is poorly known, and estimates of its size decreased by roughly an order of magnitude per decade since it was first recognized that clathrates could exist in the oceans during the 1960s and 1970s.[36] The highest estimates (e.g. 3×1018 m3)[37] were based on the assumption that fully dense clathrates could litter the entire floor of the deep ocean. Improvements in our understanding of clathrate chemistry and sedimentology have revealed that hydrates form in only a narrow range of depths (continental shelves), at only some locations in the range of depths where they could occur (10-30% of the Gas hydrate stability zone), and typically are found at low concentrations (0.9–1.5% by volume) at sites where they do occur. Recent estimates constrained by direct sampling suggest the global inventory occupies between 1×1015 and 5×1015 cubic metres (0.24 and 1.2 million cubic miles).[36] This estimate, corresponding to 500–2500 gigatonnes carbon (Gt C), is smaller than the 5000 Gt C estimated for all other geo-organic fuel reserves but substantially larger than the ~230 Gt C estimated for other natural gas sources.[36][38] The permafrost reservoir has been estimated at about 400 Gt C in the Arctic,[39][citation needed] but no estimates have been made of possible Antarctic reservoirs. These are large amounts. In comparison, the total carbon in the atmosphere is around 800 gigatons (see Carbon: Occurrence).
These modern estimates are notably smaller than the 10,000 to 11,000 Gt C (2×1016 m3) proposed[40] by previous researchers as a reason to consider clathrates to be a geo-organic fuel resource (MacDonald 1990, Kvenvolden 1998). Lower abundances of clathrates do not rule out their economic potential, but a lower total volume and apparently low concentration at most sites[36] does suggest that only a limited percentage of clathrates deposits may provide an economically viable resource.
Continental
Methane clathrates in continental rocks are trapped in beds of sandstone or siltstone at depths of less than 800 m. Sampling indicates they are formed from a mix of thermally and microbially derived gas from which the heavier hydrocarbons were later selectively removed. These occur in Alaska, Siberia, and Northern Canada.
In 2008, Canadian and Japanese researchers extracted a constant stream of natural gas from a test project at the Mallik gas hydrate site in the Mackenzie River delta. This was the second such drilling at Mallik: the first took place in 2002 and used heat to release methane. In the 2008 experiment, researchers were able to extract gas by lowering the pressure, without heating, requiring significantly less energy.[41] The Mallik gas hydrate field was first discovered by Imperial Oil in 1971–1972.[42]
Commercial use
Economic deposits of hydrate are termed natural gas hydrate (NGH) and store 164 m3 of methane, 0.8 m3 water in 1 m3 hydrate.[43] Most NGH is found beneath the seafloor (95%) where it exists in thermodynamic equilibrium. The sedimentary methane hydrate reservoir probably contains 2–10 times the currently known reserves of conventional natural gas, (As of 2013).[44] This represents a potentially important future source of hydrocarbon fuel. However, in the majority of sites deposits are thought to be too dispersed for economic extraction.[36] Other problems facing commercial exploitation are detection of viable reserves and development of the technology for extracting methane gas from the hydrate deposits.
In August 2006, China announced plans to spend 800 million yuan (US$100 million) over the next 10 years to study natural gas hydrates.[45] A potentially economic reserve in the Gulf of Mexico may contain approximately 100 billion cubic metres (3.5×1012 cu ft) of gas.[36] Bjørn Kvamme and Arne Graue at the Institute for Physics and technology at the University of Bergen have developed a method for injecting CO
2 into hydrates and reversing the process; thereby extracting CH4 by direct exchange.[46] The University of Bergen's method is being field tested by ConocoPhillips and state-owned Japan Oil, Gas and Metals National Corporation (JOGMEC), and partially funded by the U.S. Department of Energy. The project has already reached injection phase and was analyzing resulting data by March 12, 2012.[47]
On March 12, 2013, JOGMEC researchers announced that they had successfully extracted natural gas from frozen methane hydrate.[48] In order to extract the gas, specialized equipment was used to drill into and depressurize the hydrate deposits, causing the methane to separate from the ice. The gas was then collected and piped to surface where it was ignited to prove its presence.[49] According to an industry spokesperson, "It [was] the world's first offshore experiment producing gas from methane hydrate".[48] Previously, gas had been extracted from onshore deposits, but never from offshore deposits which are much more common.[49] The hydrate field from which the gas was extracted is located 50 kilometres (31 mi) from central Japan in the Nankai Trough, 300 metres (980 ft) under the sea.[48][49] A spokesperson for JOGMEC remarked "Japan could finally have an energy source to call its own".[49] Marine geologist Mikio Satoh remarked "Now we know that extraction is possible. The next step is to see how far Japan can get costs down to make the technology economically viable."[49] Japan estimates that there are at least 1.1 trillion cubic meters of methane trapped in the Nankai Trough, enough to meet the country's needs for more than ten years.[49]
Both Japan and China announced in May 2017 a breakthrough for mining methane clathrates, when they extracted methane from hydrates in the South China Sea.[14] China described the result as a breakthrough; Praveen Linga from the Department of Chemical and Biomolecular Engineering at the National University of Singapore agreed "Compared with the results we have seen from Japanese research, the Chinese scientists have managed to extract much more gas in their efforts".[50] Industry consensus is that commercial-scale production remains years away.[51]
Environmental concerns
Experts caution that environmental impacts are still being investigated and that methane—a greenhouse gas with around 25 times as much global warming potential over a 100-year period (GWP100) as carbon dioxide—could potentially escape into the atmosphere if something goes wrong.[52] Furthermore, while cleaner than coal, burning natural gas also creates carbon dioxide emissions.[53][54][55]
Hydrates in natural gas processing
Routine operations
Methane clathrates (hydrates) are also commonly formed during natural gas production operations, when liquid water is condensed in the presence of methane at high pressure. It is known that larger hydrocarbon molecules like ethane and propane can also form hydrates, although longer molecules (butanes, pentanes) cannot fit into the water cage structure and tend to destabilise the formation of hydrates.
Once formed, hydrates can block pipeline and processing equipment. They are generally then removed by reducing the pressure, heating them, or dissolving them by chemical means (methanol is commonly used). Care must be taken to ensure that the removal of the hydrates is carefully controlled, because of the potential for the hydrate to undergo a phase transition from the solid hydrate to release water and gaseous methane at a high rate when the pressure is reduced. The rapid release of methane gas in a closed system can result in a rapid increase in pressure.[15]
It is generally preferable to prevent hydrates from forming or blocking equipment. This is commonly achieved by removing water, or by the addition of ethylene glycol (MEG) or methanol, which act to depress the temperature at which hydrates will form. In recent years, development of other forms of hydrate inhibitors have been developed, like Kinetic Hydrate Inhibitors (increasing the required sub-cooling which hydrates require to form, at the expense of increased hydrate formation rate) and anti-agglomerates, which do not prevent hydrates forming, but do prevent them sticking together to block equipment.
Effect of hydrate phase transition during deep water drilling
When drilling in oil- and gas-bearing formations submerged in deep water, the reservoir gas may flow into the well bore and form gas hydrates owing to the low temperatures and high pressures found during deep water drilling. The gas hydrates may then flow upward with drilling mud or other discharged fluids. When the hydrates rise, the pressure in the annulus decreases and the hydrates dissociate into gas and water. The rapid gas expansion ejects fluid from the well, reducing the pressure further, which leads to more hydrate dissociation and further fluid ejection. The resulting violent expulsion of fluid from the annulus is one potential cause or contributor to the "kick".[56] (Kicks, which can cause blowouts, typically do not involve hydrates: see Blowout: formation kick).
Measures which reduce the risk of hydrate formation include:
- High flow-rates, which limit the time for hydrate formation in a volume of fluid, thereby reducing the kick potential.[56]
- Careful measuring of line flow to detect incipient hydrate plugging.[56]
- Additional care in measuring when gas production rates are low and the possibility of hydrate formation is higher than at relatively high gas flow rates.[56]
- Monitoring of well casing after it is "shut in" (isolated) may indicate hydrate formation. Following "shut in", the pressure rises while gas diffuses through the reservoir to the bore hole; the rate of pressure rise exhibit a reduced rate of increase while hydrates are forming.[56]
- Additions of energy (e.g., the energy released by setting cement used in well completion) can raise the temperature and convert hydrates to gas, producing a "kick".
Blowout recovery
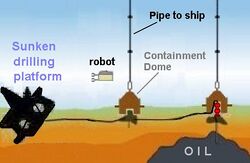
At sufficient depths, methane complexes directly with water to form methane hydrates, as was observed during the Deepwater Horizon oil spill in 2010. BP engineers developed and deployed a subsea oil recovery system over oil spilling from a deepwater oil well 5,000 feet (1,500 m) below sea level to capture escaping oil. This involved placing a 125-tonne (276,000 lb) dome over the largest of the well leaks and piping it to a storage vessel on the surface.[57] This option had the potential to collect some 85% of the leaking oil but was previously untested at such depths.[57] BP deployed the system on May 7–8, but it failed due to buildup of methane clathrate inside the dome; with its low density of approximately 0.9 g/cm3 the methane hydrates accumulated in the dome, adding buoyancy and obstructing flow.[58]
Methane clathrates and climate change
Natural gas hydrates for gas storage and transportation
Since methane clathrates are stable at a higher temperature than liquefied natural gas (LNG) (−20 vs −162 °C), there is some interest in converting natural gas into clathrates (Solidified Natural Gas or SNG) rather than liquifying it when transporting it by seagoing vessels. A significant advantage would be that the production of natural gas hydrate (NGH) from natural gas at the terminal would require a smaller refrigeration plant and less energy than LNG would. Offsetting this, for 100 tonnes of methane transported, 750 tonnes of methane hydrate would have to be transported; since this would require a ship of 7.5 times greater displacement, or require more ships, it is unlikely to prove economically feasible.[citation needed]. Recently, methane hydrate has received considerable interest for large scale stationary storage application due to the very mild storage conditions with the inclusion of tetrahydrofuran (THF) as a co-guest.[59][60] With the inclusion of tetrahydrofuran, though there is a slight reduction in the gas storage capacity, the hydrates have been demonstrated to be stable for several months in a recent study at −2 °C and atmospheric pressure.[61] A recent study has demonstrated that SNG can be formed directly with seawater instead of pure water in combination with THF.[62]
See also
- Future energy development
- Long-term effects of global warming
- The Swarm (Schätzing novel)
- Unconventional (oil & gas) reservoir
Notes
- ↑ The average methane clathrate hydrate composition is 1 mole of methane for every 5.75 moles of water. The observed density is around 0.9 g/cm3.[15] For one mole of methane, which has a molar mass of about 16.043 g (see Methane), we have 5.75 moles of water, with a molar mass of about 18.015 g (see Properties of water), so together for each mole of methane the clathrate complex has a mass of 16.043 g + 5.75 × 18.015 g ≈ 119.631 g. The fractional contribution of methane to the mass is then equal to 16.043 g / 119.631 g ≈ 0.1341. The density is around 0.9 g/cm3, so one litre of methane clathrate has a mass of around 0.9 kg, and the mass of the methane contained therein is then about 0.1341 × 0.9 kg ≈ 0.1207 kg. At a density as a gas of 0.716 kg/m3 (at 0 °C; see the info box at Methane), this comes to a volume of 0.1207 / 0.716 m3 = 0.1686 m3 = 168.6 L.
References
- ↑ Gas Hydrate: What is it?, U.S. Geological Survey, 31 August 2009, https://woodshole.er.usgs.gov/project-pages/hydrates/what.html, retrieved 28 December 2014
- ↑ 2.0 2.1 Hassan, Hussein; Romanos, Jimmy (2023-08-09). "Effects of Sea Salts on the Phase Behavior and Synthesis of Methane Hydrates + THF: An Experimental and Theoretical Study" (in en). Industrial & Engineering Chemistry Research 62 (31): 12305–12314. doi:10.1021/acs.iecr.3c00351. ISSN 0888-5885. https://pubs.acs.org/doi/10.1021/acs.iecr.3c00351.
- ↑ Sánchez, M.; Santamarina, C.; Teymouri, M.; Gai, X. (2018). "Coupled Numerical Modeling of Gas Hydrate-BearingSediments: From Laboratory to Field-Scale Analyses". Journal of Geophysical Research: Solid Earth 123 (12): 10,326-10,348. doi:10.1029/2018JB015966. Bibcode: 2018JGRB..12310326S. https://repository.kaust.edu.sa/bitstream/10754/630330/1/S-nchez_et_al-2018-Journal_of_Geophysical_Research__Solid_Earth.pdf.
- ↑ Teymouri, M.; Sánchez, M.; Santamarina, C. (2020). "A pseudo-kinetic model to simulate phase changes in gas hydrate bearing sediments". Marine and Petroleum Geology 120: 104519. doi:10.1016/j.marpetgeo.2020.104519. Bibcode: 2020MarPG.12004519T.
- ↑ Chong, Z. R.; Yang, S. H. B.; Babu, P.; Linga, P.; Li, X.-S. (2016). "Review of natural gas hydrates as an energy resource: Prospects and challenges". Applied Energy 162: 1633–1652. doi:10.1016/j.apenergy.2014.12.061.
- ↑ Hassanpouryouzband, Aliakbar; Joonaki, Edris; Vasheghani Farahani, Mehrdad; Takeya, Satoshi; Ruppel, Carolyn; Yang, Jinhai; J. English, Niall; M. Schicks, Judith et al. (2020). "Gas hydrates in sustainable chemistry". Chemical Society Reviews 49 (15): 5225–5309. doi:10.1039/C8CS00989A. PMID 32567615.
- ↑ Roald Hoffmann (2006). "Old Gas, New Gas". American Scientist 94 (1): 16–18. doi:10.1511/2006.57.3476. http://www.americanscientist.org/issues/pub/old-gas-new-gas.
- ↑ Lüthi, D; Le Floch, M; Bereiter, B; Blunier, T; Barnola, JM; Siegenthaler, U; Raynaud, D; Jouzel, J et al. (2008). "High resolution carbon dioxide concentration record 650,000–800,000 years before present". Nature 453 (7193): 379–382. doi:10.1038/nature06949. PMID 18480821. Bibcode: 2008Natur.453..379L. https://epic.awi.de/id/eprint/18281/1/Lth2008a.pdf.
- ↑ Wallmann (2018). "Gas hydrate dissociation off Svalbard induced by isostatic rebound rather than global warming". Nature Communications 9 (1): 83. doi:10.1038/s41467-017-02550-9. PMID 29311564. Bibcode: 2018NatCo...9...83W.
- ↑ Mau, S.; Römer, M.; Torres, M. E.; Bussmann, I.; Pape, T.; Damm, E.; Geprägs, P.; Wintersteller, P. et al. (23 February 2017). "Widespread methane seepage along the continental margin off Svalbard - from Bjørnøya to Kongsfjorden". Scientific Reports 7: 42997. doi:10.1038/srep42997. PMID 28230189. Bibcode: 2017NatSR...742997M.
- ↑ Pohlman, John W.; Greinert, Jens; Ruppel, Carolyn; Silyakova, Anna; Vielstädte, Lisa; Casso, Michael; Mienert, Jürgen; Bünz, Stefan (1 February 2020). "Enhanced CO2 uptake at a shallow Arctic Ocean seep field overwhelms the positive warming potential of emitted methane". Biological Sciences 114 (21): 5355–5360. doi:10.1073/pnas.1618926114. PMID 28484018.
- ↑ Fox-Kemper, B.; Hewitt, H.T.; Xiao, C.; Aðalgeirsdóttir, G.; Drijfhout, S.S.; Edwards, T.L.; Golledge, N.R.; Hemer, M. et al. (2021). Masson-Delmotte, V.; Zhai, P.; Pirani, A. et al.. eds. "Chapter 5: Global Carbon and other Biogeochemical Cycles and Feedbacks". Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, Cambridge, UK and New York, NY, USA): 5. doi:10.1017/9781009157896.011. https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Full_Report.pdf.
- ↑ Schellnhuber, Hans Joachim; Winkelmann, Ricarda; Scheffer, Marten; Lade, Steven J.; Fetzer, Ingo; Donges, Jonathan F.; Crucifix, Michel; Cornell, Sarah E. et al. (2018). "Trajectories of the Earth System in the Anthropocene". Proceedings of the National Academy of Sciences 115 (33): 8252–8259. doi:10.1073/pnas.1810141115. ISSN 0027-8424. PMID 30082409. Bibcode: 2018PNAS..115.8252S.
- ↑ 14.0 14.1 14.2 "China claims breakthrough in mining 'flammable ice'". BBC. May 19, 2017. https://www.bbc.com/news/world-asia-china-39971667.
- ↑ 15.0 15.1 15.2 Max, Michael D. (2003). Natural Gas Hydrate in Oceanic and Permafrost Environments. Kluwer Academic Publishers. p. 62. ISBN 978-0-7923-6606-5. https://books.google.com/books?id=fd8QFKwcSskC.
- ↑ Dec, Steven F.; Bowler, Kristin E.; Stadterman, Laura L.; Koh, Carolyn A.; Sloan, E. Dendy (2006). "Direct Measure of the Hydration Number of Aqueous Methane". J. Am. Chem. Soc. 128 (2): 414–415. doi:10.1021/ja055283f. PMID 16402820. Note: the number 20 is called a magic number equal to the number found for the amount of water molecules surrounding a hydronium ion.
- ↑ Guggenheim, S; Koster van Groos AF (2003). "New gas-hydrate phase: Synthesis and stability of clay-methane hydrate intercalate". Geology 31 (7): 653–656. doi:10.1130/0091-7613(2003)031<0653:NGPSAS>2.0.CO;2. Bibcode: 2003Geo....31..653G.
- ↑ Vanneste, M. et al. (2001). "Multi-frequency seismic study of gas hydrate-bearing sediments in Lake Baikal, Siberia". Marine Geology 172 (1–2): 1–21. doi:10.1016/S0025-3227(00)00117-1. Bibcode: 2001MGeol.172....1V.
- ↑ 19.0 19.1 19.2 Kvenvolden, K. (1995). "A review of the geochemistry of methane in natural gas hydrate". Organic Geochemistry 23 (11–12): 997–1008. doi:10.1016/0146-6380(96)00002-2. Bibcode: 1995OrGeo..23..997K. http://www.che.ncsu.edu/ILEET/CHE596web_Fall2011/resources/natural-gas/Methane%20Hydrate-Geochemistry.pdf. Retrieved 28 December 2014.
- ↑ Mishra, C. K.; Dewangan, P; Mukhopadhyay, R; Banerjee, D (August 2021). "Available online 7 May 2021 1875-5100/© 2021 Elsevier B.V. All rights reserved. Velocity modeling and attribute analysis to understand the gas hydrates and free gas system in the Mannar Basin, India". Journal of Natural Gas Science and Engineering 92: 104007. doi:10.1016/j.jngse.2021.104007. https://www.sciencedirect.com/science/article/pii/S1875510021002134.
- ↑ Sloan, E. Dendy (2008). Clathrate hydrates of natural gases.. Carolyn A. Koh (3rd ed.). Boca Raton, FL: CRC Press. ISBN 978-1-4200-0849-4. OCLC 85830708. https://www.worldcat.org/oclc/85830708.
- ↑ Mishra, C K; Dewangan, P; Sriram, G; Kumar, A; Dakara, G (2020). "Spatial distribution of gas hydrate deposits in Krishna-Godavari offshore basin, Bay of Bengal". Marine and Petroleum Geology 112: 104037. doi:10.1016/j.marpetgeo.2019.104037. Bibcode: 2020MarPG.11204037M.
- ↑ Kvenvolden, K A (1993). "Gas hydrates-geological perspective and global change". Reviews of Geophysics 31 (2): 173–187. doi:10.1029/93RG00268. Bibcode: 1993RvGeo..31..173K. https://doi.org/10.1029/93RG00268.
- ↑ Ruppel, Carolyn, Methane Hydrates and the Future of Natural Gas, Gas Hydrates Project, Woods Hole, MA: U.S. Geological Survey, https://mitei.mit.edu/system/files/Supplementary_Paper_SP_2_4_Hydrates.pdf, retrieved 28 December 2014
- ↑ 25.0 25.1 Dickens, GR; Paull CK; Wallace P (1997). "Direct measurement of in situ methane quantities in a large gas-hydrate reservoir". Nature 385 (6615): 426–428. doi:10.1038/385426a0. Bibcode: 1997Natur.385..426D. https://deepblue.lib.umich.edu/bitstream/2027.42/62828/1/385426a0.pdf.
- ↑ Leslie R. Sautter. "A Profile of the Southeast U.S. Continental Margin". NOAA Ocean Explorer. National Oceanic and Atmospheric Administration (NOAA). http://oceanexplorer.noaa.gov/explorations/04etta/background/profile/profile.html.
- ↑ Kvenvolden, 1998(incomplete ref)
- ↑ Snyder, Glen T.; Matsumoto, Ryo; Suzuki, Yohey; Kouduka, Mariko; Kakizaki, Yoshihiro; Zhang, Naizhong; Tomaru, Hitoshi; Sano, Yuji et al. (2020-02-05). "Evidence in the Japan Sea of microdolomite mineralization within gas hydrate microbiomes" (in en). Scientific Reports 10 (1): 1876. doi:10.1038/s41598-020-58723-y. ISSN 2045-2322. PMID 32024862. Bibcode: 2020NatSR..10.1876S.
- ↑ Kvenvolden, 1993(incomplete ref)
- ↑ Dickens 1995 (incomplete ref)
- ↑ Snyder, Glen T.; Sano, Yuji; Takahata, Naoto; Matsumoto, Ryo; Kakizaki, Yoshihiro; Tomaru, Hitoshi (2020-03-05). "Magmatic fluids play a role in the development of active gas chimneys and massive gas hydrates in the Japan Sea" (in en). Chemical Geology 535: 119462. doi:10.1016/j.chemgeo.2020.119462. ISSN 0009-2541. Bibcode: 2020ChGeo.53519462S.
- ↑ Matsumoto, R. (1995). "Causes of the δ13C anomalies of carbonates and a new paradigm 'Gas Hydrate Hypothesis'". J. Geol. Soc. Japan 101 (11): 902–924. doi:10.5575/geosoc.101.902.
- ↑ Matsumoto, R.; Watanabe, Y.; Satoh, M.; Okada, H.; Hiroki, Y.; Kawasaki, M. (1996). ODP Leg 164 Shipboard Scientific Party. "Distribution and occurrence of marine gas hydrates - preliminary results of ODP Leg 164: Blake Ridge Drilling". J. Geol. Soc. Japan 102 (11): 932–944. doi:10.5575/geosoc.102.932.
- ↑ "Clathrates - little known components of the global carbon cycle". Ethomas.web.wesleyan.edu. 2000-04-13. http://ethomas.web.wesleyan.edu/ees123/clathrate.htm.
- ↑ "Domes of frozen methane may be warning signs for new blow-outs". Phys.org. 2017. https://phys.org/news/2017-06-domes-frozen-methane-blow-outs.html.
- ↑ 36.0 36.1 36.2 36.3 36.4 36.5 Milkov, AV (2004). "Global estimates of hydrate-bound gas in marine sediments: how much is really out there?". Earth-Science Reviews 66 (3–4): 183–197. doi:10.1016/j.earscirev.2003.11.002. Bibcode: 2004ESRv...66..183M.
- ↑ Trofimuk, A. A.; N. V. Cherskiy; V. P. Tsarev (1973). "[Accumulation of natural gases in zones of hydrate—formation in the hydrosphere]" (in ru). Doklady Akademii Nauk SSSR 212: 931–934.
- ↑ USGS World Energy Assessment Team, 2000. US Geological Survey world petroleum assessment 2000––description and results. USGS Digital Data Series DDS-60.
- ↑ MacDonald, G. J. (1990). "Role of methane clathrates in past and future climates". Climatic Change 16 (3): 247–281. doi:10.1007/bf00144504. Bibcode: 1990ClCh...16..247M.
- ↑ Buffett, Bruce; David Archer (15 November 2004). "Global inventory of methane clathrate: sensitivity to changes in the deep ocean". Earth and Planetary Science Letters 227 (3–4): 185–199. doi:10.1016/j.epsl.2004.09.005. Bibcode: 2004E&PSL.227..185B. http://geosci.uchicago.edu/~archer/reprints/buffett.2004.clathrates.pdf. "Preferred ... global estimate of 318 g ... Estimates of the global inventory of methane clathrate may exceed 1019 g of carbon".
- ↑ Thomas, Brodie (2008-03-31). "Researchers extract methane gas from under permafrost". Northern News Services. http://www.nnsl.com/northern-news-services/stories/papers/mar31_08ma.html.
- ↑ "Geological Survey of Canada, Mallik 2002". Natural Resources Canada. 2007-12-20. http://gsc.nrcan.gc.ca/gashydrates/mallik2002/index_e.php.
- ↑ Max, Michael D.; Johnson, Arthur H. (2016-01-01). "Economic Characteristics of Deepwater Natural Gas Hydrate" (in en). Exploration and Production of Oceanic Natural Gas Hydrate. Springer International Publishing. pp. 39–73. doi:10.1007/978-3-319-43385-1_2. ISBN 9783319433844.
- ↑ Mann, Charles C. (April 2013). "What If We Never Run Out of Oil?". The Atlantic Monthly. https://www.theatlantic.com/magazine/archive/2013/05/what-if-we-never-run-out-of-oil/309294/. Retrieved 23 May 2013.
- ↑ "Agreements to boost bilateral ties". Chinadaily.com.cn. 2006-08-25. http://www.chinadaily.com.cn/bizchina/2006-08/25/content_674169_2.htm.
- ↑ "Norske forskere bak energirevolusjon, VB nett, in Norwegian". Vg.no. May 2007. http://www.vg.no/pub/vgart.hbs?artid=184534.
- ↑ "The National Methane Hydrates R&D Program DOE/NETL Methane Hydrate Projects". Netl.doe.gov. 2013-02-19. http://www.netl.doe.gov/technologies/oil-gas/FutureSupply/MethaneHydrates/projects/DOEProjects/MH_06553HydrateProdTrial.html.
- ↑ 48.0 48.1 48.2 "Japan extracts gas from methane hydrate in world first". BBC. March 12, 2013. https://www.bbc.co.uk/news/business-21752441.
- ↑ 49.0 49.1 49.2 49.3 49.4 49.5 Hiroko Tabuchi (March 12, 2013). "An Energy Coup for Japan: 'Flammable Ice'". New York Times. https://www.nytimes.com/2013/03/13/business/global/japan-says-it-is-first-to-tap-methane-hydrate-deposit.html.
- ↑ "China claims breakthrough in 'flammable ice'". BBC News. 2017-05-19. https://www.bbc.com/news/world-asia-china-39971667.
- ↑ "China and Japan find way to extract 'combustible ice' from seafloor, harnessing a legendary frozen fossil fuel". 19 May 2017. http://news.nationalpost.com/news/world/china-japan-extracts-combustible-ice-from-seafloor-a-step-towards-harnessing-a-legendary-frozen-fossil-fuel.
- ↑ Hausman, Sandy (2018-05-31). "Fire and ice: The untapped fossil fuel that could save or ruin our climate" (in en-GB). https://www.dw.com/en/fire-and-ice-the-untapped-fossil-fuel-that-could-save-or-ruin-our-climate/a-43246890.
- ↑ Macfarlane, Alec (19 May 2017). "China makes 'flammable ice' breakthrough in South China Sea". CNNMoney. https://money.cnn.com/2017/05/19/news/china-flammable-ice-sea/index.html.
- ↑ Anderson, Richard (17 April 2014). "Methane hydrate: Dirty fuel or energy saviour?". BBC News. https://www.bbc.com/news/business-27021610.
- ↑ Dean, Signe (23 May 2017). "China Just Extracted Gas From 'Flammable Ice', And It Could Lead to a Brand New Energy Source" (in en-gb). ScienceAlert. https://www.sciencealert.com/china-has-just-tapped-into-natural-gas-found-in-flammable-ice.
- ↑ 56.0 56.1 56.2 56.3 56.4 Wang, Zhiyuan; Sun Baojiang (2009). "Annular multiphase flow behavior during deep water drilling and the effect of hydrate phase transition". Petroleum Science 6 (1): 57–63. doi:10.1007/s12182-009-0010-3. Bibcode: 2009PetSc...6...57W.
- ↑ 57.0 57.1 Winning, David (2010-05-03). "US Oil Spill Response Team: Plan To Deploy Dome In 6–8 Days". Wall Street Journal (Dow Jones & Company). https://online.wsj.com/article/BT-CO-20100503-700843.html?mod=WSJ_latestheadlines.
- ↑ Cressey, Daniel (10 May 2010). "Giant dome fails to fix Deepwater Horizon oil disaster". Nature.com. http://blogs.nature.com/news/thegreatbeyond/2010/05/_giant_dome_fails_to_fix_deepw.html.
- ↑ Veluswamy, Hari Prakash; Wong, Alison Jia Hui; Babu, Ponnivalavan; Kumar, Rajnish; Kulprathipanja, Santi; Rangsunvigit, Pramoch; Linga, Praveen (2016). "Rapid methane hydrate formation to develop a cost effective large scale energy storage system". Chemical Engineering Journal 290: 161–173. doi:10.1016/j.cej.2016.01.026.
- ↑ Veluswamy, Hari Prakash; Kumar, Asheesh; Seo, Yutaek; Lee, Ju Dong; Linga, Praveen (2018). "A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates". Applied Energy 216: 262–285. doi:10.1016/j.apenergy.2018.02.059.
- ↑ Kumar, Asheesh; Veluswamy, Hari Prakash; Linga, Praveen; Kumar, Rajnish (2019). "Molecular level investigations and stability analysis of mixed methane-tetrahydrofuran hydrates: Implications to energy storage". Fuel 236: 1505–1511. doi:10.1016/j.fuel.2018.09.126.
- ↑ Kumar, Asheesh; Veluswamy, Hari Prakash; Kumar, Rajnish; Linga, Praveen (2019). "Direct use of seawater for rapid methane storage via clathrate (SII) hydrates". Applied Energy 235: 21–30. doi:10.1016/j.apenergy.2018.10.085.
External links
- Are there deposits of methane under the sea? Will global warming release the methane to the atmosphere? (2007)
- Methane seeps from Arctic sea bed (BBC)
- Bubbles of warming, beneath the ice (LA Times 2009)
- online calculator : hydrate formation conditions with different EOSs
Research
- Centre for Arctic Gas Hydrate, Environment and Climate (CAGE)
- Center for Hydrate Research
- USGS Geological Research Activities with U.S. Minerals Management Service - Methane Gas Hydrates
- Carbon Neutral Methane Energy Production from Hydrate Deposits (Columbia University)
Video
 |