Biology:Sortase B
| Sortase B | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 3.4.22.71 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Sortases are membrane anchored enzyme that sort these surface proteins onto the bacterial cell surface and anchor them to the peptidoglycan.[1] There are different types of sortases and each catalyse the anchoring of different proteins to cell walls.[2]
It is very important for bacteria to acquire iron during infection,[3] Iron is perhaps the most important micronutrient required for bacteria to proliferate and cause disease. Sortase B, is a 246 amino acids polypeptide with putative N-terminal membrane anchor and an active site cysteine located within the TLXTC signature motif of sortases.[4][5]
It appears these enzymes are dedicated to helping the bacteria acquire iron by anchoring iron acquisition proteins to the cell membrane[6][7] Sortase B recognises and cleaves the NPQTN motif.[8][9] It links IsDC to mature assemble peptidoglycan,[10] The enzyme catalyses a cell wall sorting reaction in which a surface protein with a sorting signal containing a NXTN motif is cleaved.
This enzyme belongs to the peptidase family C60.
Structure
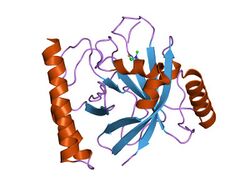
SrtB overall structure is conserved in different gram-positive bacteria. The overall structure of SrtB in S. aureus as shown the figure, consists of a unique eight stranded β-barrel core structure and a two helix subdomain at the N-terminal end.
SrtB is similar in structure to SrtA with rmsd of 1.25Å but SrtB have a more peripheral helices[6] It has an N-terminal helical bundle and an α-helix between β6 and β7. The N-terminal extension present in SrtB relative to SrtA is very significant. It is known to place the two termini on the same side of the protein. This is believed to result in a different orientation of the protein on the surface of the cell, potentially affecting substrate access.[6]
Catalysis
The sortase B enzyme catalyzes a cell wall sorting reaction with a surface protein where a signal NXTN motif is cleaved. In the result, C-end of the protein is covalently attached to a pentaglycine cross-bridge through an amide linkage, thus tethering the C-terminus of protein A to the cell wall.[11]
It cleaves the protein precursor molecule at the NPQTN motif. The peptide bond between T and N of NPQTN sorting motif is cleaved to form a tetrahedral acyl intermediate. The amino groups of the pentaglycine cross-bridges linked to the lipid II peptidoglycan precursor molecules is thought to function as a nucleophile resolving acyl intermediates and creating an amide bond between the surface protein and lipid II with subsequent incorporation of this intermediate into the cell wall envelope.
IsDC remains buried in the within the cell wall not surface located like IsDA and IsDB anchored by Sortase A. This whole system work together to scavenge iron from haemoglobin.[9]
Biological role
Surface proteins of Gram-positive bacteria play an important role in the pathogenesis of human infections such as Clostridium difficile infection.[7][1] These surface/adhesion proteins mediate the initial attachment of bacteria to host tissues. These proteins are covalently linked to the peptidoglycan of the bacterial cell wall. As more and more pathogens become resistant to antibiotics, inhibition of sortases may offer a novel strategy against gram-positive bacterial infections.[12]
SrtB in particular has gained much attention and is recognized as a promising target[13] and deletion of its gene in gram-positive bacteria will lead to serious virulence defects. Crystal structures of these SrtB enzymes from different species has been solved with ligands/inhibitors bound to their active site. With knowledge of the active site, the development of better therapeutics against these bacteria species can be done.
References
- ↑ 1.0 1.1 "Sortase, a universal target for therapeutic agents against gram-positive bacteria?". Proceedings of the National Academy of Sciences of the United States of America 97 (10): 5013–5. May 2000. doi:10.1073/pnas.97.10.5013. PMID 10805759. Bibcode: 2000PNAS...97.5013C.
- ↑ "Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Sortase catalyzed in vitro transpeptidation reaction using LPXTG peptide and NH(2)-Gly(3) substrates". The Journal of Biological Chemistry 275 (13): 9876–81. March 2000. doi:10.1074/jbc.275.13.9876. PMID 10734144. http://www.jbc.org/content/275/13/9876.
- ↑ "Surface protein IsdC and Sortase B are required for heme-iron scavenging of Bacillus anthracis". Journal of Bacteriology 188 (23): 8145–52. December 2006. doi:10.1128/JB.01011-06. PMID 17012401.
- ↑ "Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus". Molecular Microbiology 40 (5): 1049–57. June 2001. doi:10.1046/j.1365-2958.2001.02411.x. PMID 11401711. https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2958.2001.02411.x.
- ↑ "Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus". Proceedings of the National Academy of Sciences of the United States of America 98 (11): 6056–61. May 2001. doi:10.1073/pnas.101064198. PMID 11371637. Bibcode: 2001PNAS...98.6056I.
- ↑ 6.0 6.1 6.2 "Molecular features of the sortase enzyme family". The FEBS Journal 282 (11): 2097–114. June 2015. doi:10.1111/febs.13288. PMID 25845800.
- ↑ 7.0 7.1 "The structure of sortase B, a cysteine transpeptidase that tethers surface protein to the Staphylococcus aureus cell wall" (in English). Structure 12 (1): 105–12. January 2004. doi:10.1016/j.str.2003.11.021. PMID 14725770.
- ↑ "Engineering the substrate specificity of Staphylococcus aureus Sortase A. The beta6/beta7 loop from SrtB confers NPQTN recognition to SrtA". The Journal of Biological Chemistry 282 (9): 6571–81. March 2007. doi:10.1074/jbc.M610519200. PMID 17200112. http://www.jbc.org/content/282/9/6571.
- ↑ 9.0 9.1 "An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis". Proceedings of the National Academy of Sciences of the United States of America 99 (4): 2293–8. February 2002. doi:10.1073/pnas.032523999. PMID 11830639. Bibcode: 2002PNAS...99.2293M.
- ↑ "Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria". Microbiology and Molecular Biology Reviews 70 (1): 192–221. March 2006. doi:10.1128/MMBR.70.1.192-221.2006. PMID 16524923.
- ↑ "Sortase B, a new class of sortase in Listeria monocytogenes". Journal of Bacteriology 186 (7): 1972–82. April 2004. doi:10.1128/JB.186.7.1972-1982.2004. PMID 15028680.
- ↑ "Sortase enzymes in Gram-positive bacteria". Molecular Microbiology 82 (5): 1044–59. December 2011. doi:10.1111/j.1365-2958.2011.07887.x. PMID 22026821.
- ↑ "Sortase as a target of anti-infective therapy". Pharmacological Reviews 60 (1): 128–41. March 2008. doi:10.1124/pr.107.07110. PMID 18321961. https://pharmrev.aspetjournals.org/content/60/1/128.
External links
- Sortase+B at the US National Library of Medicine Medical Subject Headings (MeSH)
 |