Biology:COVID-19 drug development
COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID-19). From early 2020 through 2021, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing therapeutic candidates for COVID-19 disease in various stages of preclinical or clinical research (506 total candidates in April 2021), with 419 potential COVID-19 drugs in clinical trials, as of April 2021.[1]
As early as March 2020, the World Health Organization (WHO),[2] European Medicines Agency (EMA),[3] US Food and Drug Administration (FDA),[4] and the Chinese government and drug manufacturers[5][6] were coordinating with academic and industry researchers to speed development of vaccines, antiviral drugs, and post-infection therapies.[7][8][9][10] The International Clinical Trials Registry Platform of the WHO recorded 536 clinical studies to develop post-infection therapies for COVID-19 infections,[11][12] with numerous established antiviral compounds for treating other infections under clinical research to be repurposed.[7][13][14][15]
In March 2020, the WHO initiated the "SOLIDARITY Trial" in 10 countries, enrolling thousands of people infected with COVID-19 to assess treatment effects of four existing antiviral compounds with the most promise of efficacy.[2][16] A dynamic, systematic review was established in April 2020 to track the progress of registered clinical trials for COVID-19 vaccine and therapeutic drug candidates.[12]
Drug development is a multistep process, typically requiring more than five years to assure safety and efficacy of the new compound.[17] Several national regulatory agencies, such as the EMA and the FDA, approved procedures to expedite clinical testing.[4][18] By June 2021, dozens of potential post-infection therapies were in the final stage of human testing – phase III–IV clinical trials.[19]
Background
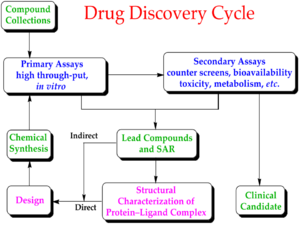
Drug development is the process of bringing a new infectious disease vaccine or therapeutic drug to the market once a lead compound has been identified through the process of drug discovery.[17] It includes laboratory research on microorganisms and animals, filing for regulatory status, such as via the FDA, for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug.[20][21] The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug normally takes more than a decade.[17][20][21]
The term "preclinical research" is defined by laboratory studies in vitro and in vivo, indicating a beginning stage for development of a preventative vaccine, antiviral or other post-infection therapies,[7] such as experiments to determine effective doses and toxicity in animals, before a candidate compound is advanced for safety and efficacy evaluation in humans.[22] To complete the preclinical stage of drug development – then be tested for safety and efficacy in an adequate number of people infected with COVID-19 (hundreds to thousands in different countries) – is a process likely to require 1–2 years for COVID-19 therapies, according to several reports in early 2020.[9][23][24][25] Despite these efforts, the success rate for drug candidates to reach eventual regulatory approval through the entire drug development process for treating infectious diseases is only 19%.[26]
Phase I trials test primarily for safety and preliminary dosing in a few dozen healthy subjects, while Phase II trials – following success in Phase I – evaluate therapeutic efficacy against the COVID-19 disease at ascending dose levels (efficacy based on biomarkers), while closely evaluating possible adverse effects of the candidate therapy (or combined therapies), typically in hundreds of people. A common trial design for Phase II studies of possible COVID-19 drugs is randomized, placebo-controlled, blinded, and conducted at multiple sites, while determining more precise, effective doses and monitoring for adverse effects.[27]
The success rate for Phase II trials to advance to Phase III (for all diseases) is about 31%, and for infectious diseases specifically, about 43%.[26] Depending on its duration (longer more expensive) – typically a period of several months to two years[27] – an average-length Phase II trial costs US$57 million (2013 dollars, including preclinical and Phase I costs).[28] Successful completion of a Phase II trial does not reliably forecast that a candidate drug will be successful in Phase III research.[29]
Phase III trials for COVID-19 involve hundreds-to-thousands of hospitalized participants, and test effectiveness of the treatment to reduce effects of the disease, while monitoring for adverse effects at the optimal dose, such as in the multinational Solidarity and Discovery trials.[2][17][30]
Candidates
| Parts of this biology (those related to section) need to be updated. Please update this biology to reflect recent events or newly available information. (February 2021) |
According to one source (as of August 2020), diverse categories of preclinical or early-stage clinical research for developing COVID-19 therapeutic candidates included:[19]
- antibodies (81 candidates)
- antivirals (31 candidates)
- cell-based compounds (34 candidates)
- RNA-based compounds (6 candidates)
- scanning compounds to be repurposed (18 candidates)
- various other therapy categories, such as anti-inflammatory, antimalarial, interferon, protein-based, antibiotics, and receptor-modulating compounds.[19]
Pivotal Phase III trials assess whether a candidate drug has efficacy specifically against a disease, and – in the case of people hospitalized with severe COVID-19 infections – test for an effective dose level of the repurposed or new drug candidate to improve the illness (primarily pneumonia) from COVID-19 infection.[2][11][32] For an already-approved drug (such as hydroxychloroquine for malaria), Phase III–IV trials determine in hundreds to thousands of COVID-19-infected people the possible extended use of an already-approved drug for treating COVID-19 infection.[32] As of August 2020, over 500 candidate therapeutics were in preclinical or a stage of Phase I–IV development, with new Phase II–III trials announced for hundreds of therapeutic candidates during 2020.[19]
Numerous candidate drugs under study as "supportive" treatments to relieve discomfort during illness, such as NSAIDs or bronchodilators, are not included in the table below. Others in early-stage Phase II trials or numerous treatment candidates in Phase I trials,[19] are also excluded. Drug candidates in Phase I–II trials have a low rate of success (under 12%) to pass through all trial phases to gain eventual approval.[20][29] Once having reached Phase III trials, therapeutic candidates for diseases related to COVID-19 infection – infectious and respiratory diseases – have a success rate of about 72%.[26]
| Drug candidate | Description | Existing disease approval | Trial sponsor(s) | Location(s) | Expected results | Notes, references |
|---|---|---|---|---|---|---|
| Remdesivir | antiviral; adenosine nucleotide analog inhibiting RNA synthesis in coronaviruses | investigational[33] | Gilead, WHO, INSERM, NIAID | China, Japan initially; extended internationally in Global Solidarity and Discovery Trials, and US NIAID ACTT Trial | Mid-2020 (Chinese, Japanese trials) | [19][34][35] selectively provided by Gilead for COVID-19 emergency access;[36][37] both promising and negative effects reported in April[38][39][40] |
| Hydroxychloroquine or chloroquine | antiparasitic and antirheumatic; generic made by many manufacturers | malaria, rheumatoid arthritis, lupus (International)[41][42] | CEPI, WHO, INSERM | Multiple sites in China; global Solidarity and Discovery Trials | June 2020 (discontinued by WHO) | multiple side effects; possible adverse prescription drug interactions;[41][42] discontinued in June from WHO Solidarity trial and UK Recovery trial as "having no clinical benefit in hospitalised patients with COVID-19";[43][44] trials[19][34] |
| Favipiravir | antiviral against influenza | influenza (China)[45] | Fujifilm | China | April 2020 | [19][8][46] |
| Lopinavir/ritonavir without or with interferon beta-1a | antiviral, immune suppression | investigational combination; lopinavir/ritonavir approved[47] | CEPI, WHO, UK Government, Univ. of Oxford, INSERM | Global Solidarity and Discovery Trials, multiple countries | mid-2020 | [19][34] |
| Sarilumab | human monoclonal antibody against interleukin-6 receptor | rheumatoid arthritis (US, Europe)[48] | Regeneron-Sanofi | Multiple countries | Spring 2020 | [19][49] |
| ASC-09 + ritonavir | antiviral | combination not approved; ritonavir approved for HIV[47] | Ascletis Pharma | Multiple sites in China | Spring 2020 | [19][50] |
| Tocilizumab | human monoclonal antibody against interleukin-6 receptor | immunosuppression, rheumatoid arthritis (US, Europe)[51] | Genentech-Hoffmann-La Roche | Multiple countries | mid-2020 | [19][52] Roche announced in late July that its Phase III trial of tocilizumab for treating pneumonia in hospitalized people with COVID-19 infection was ineffective[53] |
| Lenzilumab | humanized monoclonal antibody for relieving pneumonia | new drug candidate | Humanigen, Inc. | Multiple sites in the United States | September 2020 | [19][54] |
| Dapagliflozin | sodium-glucose cotransporter 2 inhibitor | hypoglycemia agent[55] | Saint Luke's Mid America Heart Institute, AstraZeneca | Multiple countries | December 2020 | [19][56] |
| CD24Fc | antiviral immunomodulator against inflammatory response | new drug candidate | OncoImmune, Inc. | Multiple sites in the United States | 2021 | [19][57] |
| Apabetalone | selective BET inhibitor | investigational | Resverlogix Corp | United States | 22 March 2022 | [19][58] |
Repurposed drug candidates
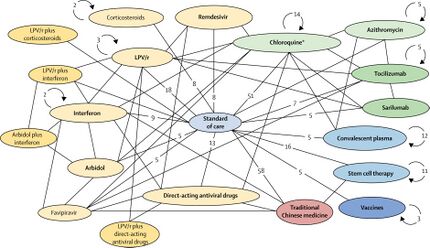
Drug repositioning (also called drug repurposing) – the investigation of existing drugs for new therapeutic purposes – is one line of scientific research followed to develop safe and effective COVID-19 treatments.[15][59] Several existing antiviral medications, previously developed or used as treatments for Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), HIV/AIDS, and malaria, are being researched as COVID-19 treatments, with some moving into clinical trials.[13]
During the COVID-19 pandemic, drug repurposing is the clinical research process of rapidly screening and defining the safety and efficacy of existing drugs already approved for other diseases to be used for people with COVID-19 infection.[13][15][60] In the usual drug development process,[17] confirmation of repurposing for new disease treatment would take many years of clinical research – including pivotal Phase III clinical trials – on the candidate drug to assure its safety and efficacy specifically for treating COVID-19 infection.[13][60] In the emergency of a growing COVID-19 pandemic, the drug repurposing process was being accelerated during March 2020 to treat people hospitalized with COVID-19.[2][13][15]
Clinical trials using repurposed, generally safe, existing drugs for hospitalized COVID-19 people may take less time and have lower overall costs to obtain endpoints proving safety (absence of serious side effects) and post-infection efficacy, and can rapidly access existing drug supply chains for manufacturing and worldwide distribution.[2][13][61] In an international effort to capture these advantages, the WHO began in mid-March 2020 expedited international Phase II–III trials on four promising treatment options – the SOLIDARITY trial[2][62][63] – with numerous other drugs having potential for repurposing in different disease treatment strategies, such as anti-inflammatory, corticosteroid, antibody, immune, and growth factor therapies, among others, being advanced into Phase II or III trials during 2020.[19][13][60][64]
In March 2020, the United States Centers for Disease Control and Prevention (CDC) issued a physician advisory concerning remdesivir for people hospitalized with pneumonia caused by COVID-19: "While clinical trials are critical to establish the safety and efficacy of this drug, clinicians without access to a clinical trial may request remdesivir for compassionate use through the manufacturer for patients with clinical pneumonia."[37]
Novel antibody drugs
Convalescent plasma
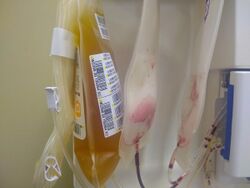
Passive immunization with convalescent plasma or hyperimmune serum has been proposed as a potential treatment for COVID-19. As of May 2021, there is strong evidence that convalescent plasma treatment is not associated with clinical improvements for people with moderate or severe disease and does not decrease the risk of dying.[65] The potential for adverse effects associated with convalescent plasma treatment is unknown.[65]
In the United States, the FDA has granted temporary authorization to convalescent plasma (plasma from the blood of people who have recovered from COVID-19, which thus contains antibodies against SARS-CoV-2) as an experimental treatment in cases where the person's life is seriously or immediately threatened.[66] As of May 2021, at least 12 randomized controlled trials on the effectiveness of convalescent plasma treatment were published in peer-reviewed medical journals.[65] In addition, as of May 2021, 100 additional trials were 'ongoing' and 33 studies were reported as 'competed' but not yet published.[65]
Argentina, Brazil, Costa Rica, and Mexico have pursued development of antisera.[67] Brazil began development of an equine hyperimmune serum, obtained by inoculating horses with recombinant SARS-CoV-2 spike protein, in mid-2020. A consortium of Instituto Vital Brazil, UFRJ, the Oswaldo Cruz Foundation and the D'Or Institute for Research and Education in Rio de Janeiro began preclinical trials in May 2020,[68] while Instituto Butantan in São Paulo completed animal testing in September.[67] In December 2020, Argentina granted emergency authorization to CoviFab, a locally developed formulation of equine hyperimmune serum, for use in cases of moderate to severe COVID-19, based on the initial results of a single phase 2/3 trial which suggested reductions in mortality, ICU admission, and mechanical ventilation requirements in patients who received the serum.[69][70] This was harshly criticized by the Argentine Intensive Care Society, which stated that the trial failed to achieve its primary or secondary endpoints and did not demonstrate any statistically significant differences between the serum and placebo groups.[70]
Bamlanivimab/etesevimab
Bebtelovimab
Casirivimab/imdevimab
Regdanvimab
Sotrovimab
Tixagevimab/cilgavimab
Vilobelimab
Novel viral replication inhibitors
Molnupiravir
Novel protease inhibitors
Ensitrelvir
Other
Sabizabulin
Planning and coordination
Early planning
Over 2018–20, new initiatives to stimulate vaccine and antiviral drug development included partnerships between governmental organizations and industry, such as the European Innovative Medicines Initiative,[71] the US Critical Path Initiative to enhance innovation of drug development,[72] and the Breakthrough Therapy designation to expedite development and regulatory review of promising candidate drugs.[73] To accelerate refinement of diagnostics for detecting COVID-19 infection, a global diagnostic pipeline tracker was formed.[74]
According to a tracker of clinical trial progress on potential therapeutic drugs for COVID-19 infections, 29 Phase II–IV efficacy trials were concluded in March 2020 or scheduled to provide results in April from hospitals in China – which experienced the first outbreak of COVID-19 in late 2019.[19] Seven trials were evaluating repurposed drugs already approved to treat malaria, including four studies on hydroxychloroquine or chloroquine phosphate.[19] Repurposed antiviral drugs make up most of the Chinese research, with 9 Phase III trials on remdesivir across several countries due to report by the end of April.[19] Other potential therapeutic candidates under pivotal clinical trials concluding in March–April are vasodilators, corticosteroids, immune therapies, lipoic acid, bevacizumab, and recombinant angiotensin-converting enzyme 2, among others.
The COVID-19 Clinical Research Coalition has goals to 1) facilitate rapid reviews of clinical trial proposals by ethics committees and national regulatory agencies, 2) fast-track approvals for the candidate therapeutic compounds, 3) ensure standardised and rapid analysis of emerging efficacy and safety data, and 4) facilitate sharing of clinical trial outcomes before publication.[11] A dynamic review of clinical development for COVID-19 vaccine and drug candidates was in place, as of April.[12]
By March 2020, the international Coalition for Epidemic Preparedness Innovations (CEPI) committed to research investments of US$100 million across several countries,[75] and issued an urgent call to raise and rapidly invest $2 billion for vaccine development.[76] Led by the Bill and Melinda Gates Foundation with partners investing US$125 million and coordinating with the World Health Organization, the COVID-19 Therapeutics Accelerator began in March, facilitating drug development researchers to rapidly identify, assess, develop, and scale up potential treatments.[77] The COVID-19 Clinical Research Coalition formed to coordinate and expedite results from international clinical trials on the most promising post-infection treatments.[11] In early 2020, numerous established antiviral compounds for treating other infections were being repurposed or developed in new clinical research efforts to alleviate the illness of COVID-19.[7][13][19]
During March 2020, the Coalition for Epidemic Preparedness Innovations (CEPI) initiated an international COVID-19 vaccine development fund, with the goal to raise US$2 billion for vaccine research and development,[78] and committed to investments of US$100 million in vaccine development across several countries.[75] The Canadian government announced CA$275 million in funding for 96 research projects on medical countermeasures against COVID-19, including numerous vaccine candidates at Canadian universities,[79][80] with plans to establish a "vaccine bank" of new vaccines for implementation if another COVID-19 outbreak occurs.[80][81] The Bill & Melinda Gates Foundation invested US$150 million in April for development of COVID-19 vaccines, diagnostics, and therapeutics.[82]
Computer-assisted research
In March 2020, the United States Department of Energy, National Science Foundation, NASA, industry, and nine universities pooled resources to access supercomputers from IBM, combined with cloud computing resources from Hewlett Packard Enterprise, Amazon, Microsoft, and Google, for drug discovery.[83][84] The COVID-19 High Performance Computing Consortium also aims to forecast disease spread, model possible vaccines, and screen thousands of chemical compounds to design a COVID-19 vaccine or therapy.[83][84] The Consortium used up 437 petaFLOPS of computing power by May 2020.[85]
The C3.ai Digital Transformation Institute, an additional consortium of Microsoft, six universities (including the Massachusetts Institute of Technology, a member of the first consortium), and the National Center for Supercomputer Applications in Illinois, working under the auspices of C3.ai, an artificial intelligence software company, are pooling supercomputer resources toward drug discovery, medical protocol development and public health strategy improvement, as well as awarding large grants to researchers who proposed by May to use AI to carry out similar tasks.[86][87]
In March 2020, the distributed computing project Folding@home launched a program to assist drug developers, initially simulating protein targets from SARS-CoV-2 and the related SARS-CoV virus, which has been studied previously.[88][89][90][91][92][93]
Distributed computing project Rosetta@home also joined the effort in March. The project uses computers of volunteers to model SARS-CoV-2 virus proteins to discover possible drug targets or create new proteins to neutralize the virus. Researchers revealed that with the help of Rosetta@home, they had been able to "accurately predict the atomic-scale structure of an important coronavirus protein weeks before it could be measured in the lab."[94]
In May 2020, the OpenPandemics – COVID-19 partnership between Scripps Research and IBM's World Community Grid was launched. The partnership is a distributed computing project that "will automatically run a simulated experiment in the background [of connected home PCs] which will help predict the effectiveness of a particular chemical compound as a possible treatment for COVID-19".[95]
International Solidarity and Discovery Trials
In March, the World Health Organization (WHO) launched the coordinated "Solidarity Trial" in 10 countries on five continents to rapidly assess in thousands of COVID-19 infected people the potential efficacy of existing antiviral and anti-inflammatory agents not yet evaluated specifically for COVID-19 illness.[2][16] By late April, hospitals in over 100 countries were involved in the trial.[96]
The individual or combined drugs undergoing initial studied are 1) lopinavir–ritonavir combined, 2) lopinavir–ritonavir combined with interferon-beta, 3) remdesivir or 4) (hydroxy)chloroquine in separate trials and hospital sites internationally.[2][16] Following a study published by The Lancet on safety concerns with hydroxychloroquine, the WHO suspended use of it from the Solidarity trial in May 2020,[97][98] reinstated it after the research was retracted,[99] then abandoned further use of the drug for COVID-19 treatment when analysis showed in June that it provided no benefit.[43]
With about 15% of people infected by COVID-19 having severe illness, and hospitals being overwhelmed during the pandemic, WHO recognized a rapid clinical need to test and repurpose these drugs as agents already approved for other diseases and recognized as safe.[2] The Solidarity project is designed to give rapid insights to key clinical questions:[2][100]
- Do any of the drugs reduce mortality?
- Do any of the drugs reduce the time a patient is hospitalized?
- Do the treatments affect the need for people with COVID-19-induced pneumonia to be ventilated or maintained in intensive care?
- Could such drugs be used to minimize the illness of COVID-19 infection in healthcare staff and people at high risk of developing severe illness?
Enrolling people with COVID-19 infection is simplified by using data entries, including informed consent, on a WHO website. After the trial staff determines the drugs available at the hospital, the WHO website randomizes the hospitalized subject to one of the trial drugs or to the hospital standard of care for treating COVID-19. The trial physician records and submits follow-up information about the subject status and treatment, completing data input via the WHO Solidarity website. The design of the Solidarity trial is not double-blind – which is normally the standard in a high-quality clinical trial – but the WHO needed speed with quality for the trial across many hospitals and countries.[2] A global safety monitoring board of WHO physicians examine interim results to assist decisions on safety and effectiveness of the trial drugs, and alter the trial design or recommend an effective therapy.[2][100] A similar web-based study to Solidarity, called "Discovery", was initiated in March across seven countries by INSERM (Paris, France).[2][34]
The Solidarity trial seeks to implement coordination across hundreds of hospital sites in different countries – including those with poorly-developed infrastructure for clinical trials – yet needs to be conducted rapidly. According to John-Arne Røttingen, chief executive of the Research Council of Norway and chairman of the Solidarity trial international steering committee, the trial would be considered effective if therapies are determined to "reduce the proportion of patients that need ventilators by, say, 20%, that could have a huge impact on our national health-care systems."[30]
During March, funding for the Solidarity trial reached US$108 million from 203,000 individuals, organizations and governments, with 45 countries involved in financing or trial management.[97]
A clinical trial design in progress may be modified as an "adaptive design" if accumulating data in the trial provide early insights about positive or negative efficacy of the treatment.[101][102] The global Solidarity and European Discovery trials of hospitalized people with severe COVID-19 infection apply adaptive design to rapidly alter trial parameters as results from the four experimental therapeutic strategies emerge.[11][34][103] Adaptive designs within ongoing Phase II–III clinical trials on candidate therapeutics may shorten trial durations and use fewer subjects, possibly expediting decisions for early termination or success, and coordinating design changes for a specific trial across its international locations.[29][102][104]
Adaptive COVID-19 Treatment Trial
The US National Institute of Allergy and Infectious Diseases (NIAID) initiated an adaptive design, international Phase III trial (called "ACTT") to involve up to 800 hospitalized COVID-19 people at 100 sites in multiple countries. Beginning with use of remdesivir as the primary treatment over 29 days, the trial definition of its adaptive protocol states that "there will be interim monitoring to introduce new arms and allow early stopping for futility, efficacy, or safety. If one therapy proves to be efficacious, then this treatment may become the control arm for comparison(s) with new experimental treatment(s)."[38]
Operation Warp Speed
RECOVERY Trial
A large-scale, randomized controlled trial named the RECOVERY Trial was set up in March 2020, in the UK to test possible treatments for COVID-19. It is run by the Nuffield Departments of Public Health and of Medicine at the University of Oxford and is testing five repurposed drugs and also convalescent plasma. The trial enrolled more than 11,500 COVID-19 positive participants in the U.K by June 2020.[44][105][106]
During April, the British RECOVERY (Randomised Evaluation of COVid-19 thERapY) trial was launched initially in 132 hospitals across the UK,[107] expanding to become one of the world's largest COVID-19 clinical studies, involving 5400 infected people under treatment at 165 UK hospitals, as of mid-April.[108] The trial is examining different potential therapies for severe COVID-19 infection: lopinavir/ritonavir, low-dose dexamethasone (an anti-inflammatory steroid), hydroxychloroquine, and azithromycin (a common antibiotic).[105] In June, the trial arm using hydroxychloroquine was discontinued when analyses showed it provided no benefit.[44]
On 16 June the trial group released a statement that dexamethasone had been shown to reduce mortality in patients receiving respiratory support.[109] In a controlled trial around 2,000 hospital patients were given dexamethasone and were compared with more than 4,000 who did not receive the drug. For patients on ventilators, it cut the risk of death from 40% to 28% (1 in 8). For patients needing oxygen, it cut the risk of death from 25% to 20% (1 in 5).[110]
By the end of June 2020, the trial had published findings regarding hydroxychloroquine and dexamethasone.[44][111] It had also announced results for lopinavir/ritonavir which were published in October 2020. The lopinavir-ritonavir and hydroxychloroquine arms were closed to new entrants after being shown to be ineffective.[44][112][113] Dexamethasone was closed to new adult entries after positive results and by November 2020, was open to child entries.
PANORAMIC trial
Launched in December 2021, the PANORAMIC trial will test the effectiveness of molnupiravir and nirmatrelvir/ritonavir in preventing hospitalisation and helping faster recovery for people aged over 50 and those at higher risk due to underlying health conditions.[114][115] PANORAMIC is sponsored by the University of Oxford and funded by the National Institute for Health Research (NIHR).[114] As of March 2022 has over 16,000 people enrolled as participants making it the largest study into COVID-19 antivirals.[116]
See also
- Cost of drug development
- COVID Moonshot
References
- ↑ "COVID-19 vaccine and therapeutics tracker". BioRender. 5 April 2021. https://biorender.com/covid-vaccine-tracker.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 "WHO launches global megatrial of the four most promising coronavirus treatments". Science Magazine. 22 March 2020. doi:10.1126/science.abb8497. https://www.science.org/content/article/who-launches-global-megatrial-four-most-promising-coronavirus-treatments. Retrieved 27 March 2020.
- ↑ "First regulatory workshop on COVID-19 facilitates global collaboration on vaccine development". European Medicines Agency. 18 March 2020. https://www.ema.europa.eu/en/news/first-regulatory-workshop-covid-19-facilitates-global-collaboration-vaccine-development.
- ↑ 4.0 4.1 "Coronavirus (COVID-19) Update: FDA Continues to Facilitate Development of Treatments" (Press release). U.S. Food and Drug Administration (FDA). 19 March 2020. Archived from the original on 20 March 2020. Retrieved 21 March 2020.
- ↑ "China approves first anti-viral drug against coronavirus Covid-19". Clinical Trials Arena. 18 February 2020. https://www.clinicaltrialsarena.com/news/china-approves-favilavir-covid-19/.
- ↑ "Chinese Vaccine Approved for Human Testing at Virus Epicenter". Bloomberg News. 19 March 2020. https://www.bloomberg.com/news/articles/2020-03-18/chinese-vaccine-approved-for-human-testing-at-virus-epicenter.
- ↑ 7.0 7.1 7.2 7.3 "COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics". Human Vaccines & Immunotherapeutics 16 (6): 1232–1238. June 2020. doi:10.1080/21645515.2020.1735227. PMID 32186952.
- ↑ 8.0 8.1 "Potential interventions for novel coronavirus in China: A systematic review". Journal of Medical Virology 92 (5): 479–490. May 2020. doi:10.1002/jmv.25707. PMID 32052466.
- ↑ 9.0 9.1 "Drug makers are racing to develop immune therapies for Covid-19. Will they be ready in time?". Stat. 19 March 2020. https://www.statnews.com/2020/03/19/coronavirus-antibody-therapies-will-they-be-ready-in-time/.
- ↑ "Chinese military scientists ordered to win global race to develop coronavirus vaccine". South China Morning Post. 19 March 2020. https://www.scmp.com/news/china/military/article/3075843/chinese-military-scientists-ordered-win-global-race-develop.
- ↑ 11.0 11.1 11.2 11.3 11.4 COVID-19 Clinical Research Coalition (April 2020). "Global coalition to accelerate COVID-19 clinical research in resource-limited settings". Lancet 395 (10233): 1322–1325. doi:10.1016/s0140-6736(20)30798-4. PMID 32247324.
- ↑ 12.0 12.1 12.2 "A living systematic review protocol for COVID-19 clinical trial registrations". Wellcome Open Research 5: 60. 2 April 2020. doi:10.12688/wellcomeopenres.15821.1. PMID 32292826.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)". Nature Reviews. Drug Discovery 19 (3): 149–150. March 2020. doi:10.1038/d41573-020-00016-0. PMID 32127666.
- ↑ "Discovering drugs to treat coronavirus disease 2019 (COVID-19)". Drug Discoveries & Therapeutics 14 (1): 58–60. 29 February 2020. doi:10.5582/ddt.2020.01012. PMID 32147628. https://www.jstage.jst.go.jp/article/ddt/14/1/14_2020.01012/_pdf/-char/en. Retrieved 21 March 2020.
- ↑ 15.0 15.1 15.2 15.3 "Coronavirus puts drug repurposing on the fast track". Nature Biotechnology 38 (4): 379–381. April 2020. doi:10.1038/d41587-020-00003-1. PMID 32205870.
- ↑ 16.0 16.1 16.2 "Generating randomized trial evidence to optimize treatment in the COVID-19 pandemic". CMAJ 192 (15): E405–E407. April 2020. doi:10.1503/cmaj.200438. PMID 32336678.
- ↑ 17.0 17.1 17.2 17.3 17.4 "The Drug Development Process". U.S. Food and Drug Administration (FDA). 4 January 2018. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process.
- ↑ "Call to pool research resources into large multi-centre, multi-arm clinical trials to generate sound evidence on COVID-19 treatments". European Medicines Agency. 19 March 2020. https://www.ema.europa.eu/en/news/call-pool-research-resources-large-multi-centre-multi-arm-clinical-trials-generate-sound-evidence.
- ↑ 19.00 19.01 19.02 19.03 19.04 19.05 19.06 19.07 19.08 19.09 19.10 19.11 19.12 19.13 19.14 19.15 19.16 19.17 19.18 19.19 19.20 "COVID-19 vaccine and treatments tracker (Choose vaccines or treatments tab, apply filters to view select data)". Milken Institute. 21 June 2021. https://airtable.com/shrSAi6t5WFwqo3GM/tblEzPQS5fnc0FHYR/viwJJuMA1ioIgAcPs?blocks=hide.
- ↑ 20.0 20.1 20.2 "Early Drug Discovery and Development Guidelines: For Academic Researchers, Collaborators, and Start-up Companies". Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences. 1 July 2016. https://www.ncbi.nlm.nih.gov/books/NBK92015/. Retrieved 21 March 2020.
- ↑ 21.0 21.1 "The Pharmaceutical Industry and the Future of Drug Development". Issues in Environmental Science and Technology (Royal Society of Chemistry): 1–33. 2015. doi:10.1039/9781782622345-00001. ISBN 978-1-78262-189-8.
- ↑ "Step 2: Preclinical Research". U.S. Food and Drug Administration (FDA). 4 January 2018. https://www.fda.gov/patients/drug-development-process/step-2-preclinical-research.
- ↑ "Here's why the WHO says a coronavirus vaccine is 18 months away". 14 February 2020. https://theconversation.com/heres-why-the-who-says-a-coronavirus-vaccine-is-18-months-away-131213.
- ↑ "Why will a coronavirus vaccine take so long?". The Boston Globe. 19 March 2020. https://www.bostonglobe.com/2020/03/19/opinion/why-will-coronavirus-vaccine-take-so-long/.
- ↑ "Responding to Covid-19 – A Once-in-a-Century Pandemic?". The New England Journal of Medicine 382 (18): 1677–1679. February 2020. doi:10.1056/nejmp2003762. PMID 32109012.
- ↑ 26.0 26.1 26.2 "Clinical development success rates: 2006–2015". BIO Industry Analysis. June 2016. https://www.bio.org/sites/default/files/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf.
- ↑ 27.0 27.1 "The drug development process: Clinical research". U.S. Food and Drug Administration (FDA). 4 January 2018. https://www.fda.gov/patients/drug-development-process/step-3-clinical-research.
- ↑ "Innovation in the pharmaceutical industry: New estimates of R&D costs". Journal of Health Economics 47: 20–33. May 2016. doi:10.1016/j.jhealeco.2016.01.012. PMID 26928437. https://dukespace.lib.duke.edu/dspace/bitstream/handle/10161/12742/DiMasi-Grabowski-Hansen-RnD-JHE-2016.pdf;sequence=1. Retrieved 24 March 2020.
- ↑ 29.0 29.1 29.2 "Phase II Trials in Drug Development and Adaptive Trial Design". JACC. Basic to Translational Science 4 (3): 428–437. June 2019. doi:10.1016/j.jacbts.2019.02.005. PMID 31312766.
- ↑ 30.0 30.1 "Flooded by the torrent: the COVID-19 drug pipeline". Lancet 395 (10232): 1245–1246. April 2020. doi:10.1016/S0140-6736(20)30894-1. PMID 32305088.
- ↑ 31.0 31.1 "A real-time dashboard of clinical trials for COVID-19". The Lancet. Digital Health 2 (6): e286–e287. June 2020. doi:10.1016/S2589-7500(20)30086-8. PMID 32363333.
- ↑ 32.0 32.1 "What are the phases of clinical trials?". American Cancer Society. 2020. https://www.cancer.org/treatment/treatments-and-side-effects/clinical-trials/what-you-need-to-know/phases-of-clinical-trials.html.
- ↑ "Remdesivir approval status". Drugs.com. 24 March 2020. https://www.drugs.com/history/remdesivir.html.
- ↑ 34.0 34.1 34.2 34.3 34.4 "Launch of a European clinical trial against COVID-19". INSERM. 22 March 2020. https://presse.inserm.fr/en/launch-of-a-european-clinical-trial-against-covid-19/38737/. "The great strength of this trial is its "adaptive" nature. This means that ineffective experimental treatments can very quickly be dropped and replaced by other molecules that emerge from research efforts. We will therefore be able to make changes in real time, in line with the most recent scientific data, in order to find the best treatment for our patients"
- ↑ "A closer look at the Ebola drug that's become the top hope for a coronavirus treatment". BioPharma Dive. 5 March 2020. https://www.biopharmadive.com/news/coronavirus-remdesivir-gilead-antiviral-drug-covid-19/573261/. "There's only one drug right now that we think may have real efficacy. And that's remdesivir." said Bruce Aylward, a senior advisor and international leader of the World Health Organization's joint mission to China"
- ↑ "Emergency access to remdesivir outside of clinical trials". Gilead Sciences. 1 April 2020. https://www.gilead.com/purpose/advancing-global-health/covid-19/emergency-access-to-remdesivir-outside-of-clinical-trials.
- ↑ 37.0 37.1 "Information for clinicians on therapeutic options for COVID-19 patients". US Centers for Disease Control and Prevention. 21 March 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html.
- ↑ 38.0 38.1 Clinical trial number NCT04280705 for "Adaptive COVID-19 Treatment Trial (ACTT)" at ClinicalTrials.gov
- ↑ "NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19" (Press release). US National Institute of Allergy and Infectious Diseases. 29 April 2020. Archived from the original on 30 April 2020. Retrieved 29 April 2020.
- ↑ "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial". Lancet 395 (10236): 1569–1578. May 2020. doi:10.1016/S0140-6736(20)31022-9. PMID 32423584.
- ↑ 41.0 41.1 "Hydroxychloroquine sulfate". Drugs.com. 31 March 2020. https://www.drugs.com/monograph/hydroxychloroquine-sulfate.html.
- ↑ 42.0 42.1 "Chloroquine phosphate". Drugs.com. 31 March 2020. https://www.drugs.com/monograph/chloroquine-phosphate.html.
- ↑ 43.0 43.1 "Hydroxychloroquine halted in WHO-sponsored COVID-19 trials". Bloomberg. 17 June 2020. https://www.bloomberg.com/news/articles/2020-06-17/hydroxychloroquine-testing-halted-in-who-sponsored-covid-trial.
- ↑ 44.0 44.1 44.2 44.3 44.4 "No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19". Recovery Trial, Nuffield Department of Population Health, University of Oxford, UK. 5 June 2020. https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19.
- ↑ "Fujifilm Announces the Start of a Phase III Clinical Trial of Influenza Antiviral Drug Avigan (favipiravir) on COVID-19 in Japan and Commits to Increasing Production". Drugs.com via Fujifilm Toyama Chemical Co., Ltd. 31 March 2020. https://www.drugs.com/clinical_trials/fujifilm-announces-start-phase-iii-clinical-trial-influenza-antiviral-avigan-favipiravir-covid-19-18505.html.
- ↑ "Coronavirus: Japanese anti-viral drug effective in treating patients, Chinese official says". The Independent. 18 March 2020. https://www.independent.co.uk/news/world/asia/coronavirus-treatment-anti-viral-drug-favipiravir-avigan-wuhan-china-a9408066.html.
- ↑ 47.0 47.1 "Ritonavir". Drugs.com. 2020. https://www.drugs.com/international/ritonavir.html.
- ↑ "Kevzara". Drugs.com. 7 March 2019. https://www.drugs.com/kevzara.html.
- ↑ "Sanofi begins trial of Kevzara against COVID-19 complications". PharmaPhorum. 31 March 2020. https://pharmaphorum.com/news/sanofi-begins-trial-of-kevzara-against-covid-19-complications/.
- ↑ "All the COVID-19 vaccines and treatments currently in clinical trials". Digital Trends. 2 April 2020. https://www.digitaltrends.com/health-fitness/coronavirus-covid-19-vaccines-treatments-list/.
- ↑ "Tocilizumab". Drugs.com. 7 June 2019. https://www.drugs.com/mtm/tocilizumab.html.
- ↑ "FDA approves Phase III clinical trial of tocilizumab for COVID-19 pneumonia". Cancer Network, MJH Life Sciences. 26 March 2020. https://www.cancernetwork.com/news/fda-approves-phase-iii-clinical-trial-tocilizumab-covid-19-pneumonia.
- ↑ "Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia". Hoffmann-La Roche. 29 July 2020. https://www.roche.com/media/releases/med-cor-2020-07-29.htm.
- ↑ Clinical trial number NCT04351152 for "Phase 3 Study to Evaluate Efficacy and Safety of Lenzilumab in Hospitalized Patients With COVID-19 Pneumonia" at ClinicalTrials.gov
- ↑ "Dapagliflozin: MedlinePlus Drug Information". 20 April 2020. https://medlineplus.gov/druginfo/meds/a614015.html.
- ↑ Clinical trial number NCT04350593 for "Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19)" at ClinicalTrials.gov
- ↑ Clinical trial number NCT04317040 for "CD24Fc as a Non-antiviral Immunomodulator in COVID-19 Treatment (SAC-COVID)" at ClinicalTrials.gov
- ↑ An Open-Label Study of Apabetalone in Covid Infection. 20 May 2021. https://clinicaltrials.gov/ct2/show/NCT04894266. Retrieved 24 May 2021.
- ↑ "Repurposing drugs". National Center for Advancing Translational Sciences (NCATS). 7 November 2017. https://ncats.nih.gov/preclinical/repurpose.
- ↑ 60.0 60.1 60.2 "Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China". F1000Research 9: 72. 31 January 2020. doi:10.12688/f1000research.22211.1. PMID 32117569.
- ↑ "Use of antiviral drugs to reduce COVID-19 transmission". The Lancet. Global Health (Elsevier BV) 8 (5): e639–e640. March 2020. doi:10.1016/s2214-109x(20)30114-5. PMID 32199468.
- ↑ "UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment". United Nations – News. World Health Organization. 18 March 2020. https://news.un.org/en/story/2020/03/1059722.
- ↑ "Race to find COVID-19 treatments accelerates". Science 367 (6485): 1412–1413. March 2020. doi:10.1126/science.367.6485.1412. PMID 32217705. Bibcode: 2020Sci...367.1412K.
- ↑ "COVID-19 drug development: Landscape analysis of therapeutics (table)". United Nations, World Health Organization. 21 March 2020. https://www.who.int/blueprint/priority-diseases/key-action/Table_of_therapeutics_Appendix_17022020.pdf?ua=1.
- ↑ 65.0 65.1 65.2 65.3 Piechotta, Vanessa; Iannizzi, Claire; Chai, Khai Li; Valk, Sarah J.; Kimber, Catherine; Dorando, Elena; Monsef, Ina; Wood, Erica M. et al. (20 May 2021). "Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review". The Cochrane Database of Systematic Reviews 2021 (5): CD013600. doi:10.1002/14651858.CD013600.pub4. ISSN 1469-493X. PMID 34013969.
- ↑ "FDA now allows treatment of life-threatening COVID-19 cases using blood from patients who have recovered". 24 March 2020. https://social.techcrunch.com/2020/03/24/fda-now-allows-treatment-of-life-threatening-covid-19-cases-using-blood-from-patients-who-have-recovered/.
- ↑ 67.0 67.1 "Butantan desenvolve soro contra coronavírus" (in pt). Revista Pesquisa Fapesp. 14 December 2020. https://revistapesquisa.fapesp.br/butantan-desenvolve-soro-contra-coronavirus/.
- ↑ "Vital Brazil desenvolve soro contra covid-19, mas medicamento ainda não foi testado em humanos" (in pt). O Estado de S. Paulo. 4 January 2021. https://politica.estadao.com.br/blogs/estadao-verifica/vital-brazil-desenvolve-soro-contra-covid-19-mas-medicamento-ainda-nao-foi-testado-em-humanos/.
- ↑ "Argentina aprueba uso de suero equino que reduce en un 45% las muertes por COVID-19" (in es). Deutsche Welle. 11 January 2021. https://www.dw.com/es/argentina-aprueba-uso-de-suero-equino-que-reduce-en-un-45-las-muertes-por-covid-19/a-56193399.
- ↑ 70.0 70.1 "Coronavirus: terapistas rechazaron "fuertemente" el uso de suero equino en pacientes graves" (in es). Clarín. 17 January 2021. https://www.clarin.com/sociedad/coronavirus-terapistas-rechazaron-uso-suero-equino-cuestionan-anmat_0_TjaKglS9w.html.
- ↑ "About the Innovative Medicines Initiative". European Innovative Medicines Initiative. 2020. https://www.imi.europa.eu/about-imi.
- ↑ "Critical Path Initiative". U.S. Food and Drug Administration (FDA). 23 April 2018. https://www.fda.gov/science-research/science-and-research-special-topics/critical-path-initiative.
- ↑ "Breakthrough Therapy". U.S. Food and Drug Administration (FDA). 4 January 2018. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy.
- ↑ "SARS-CoV-2 Diagnostic Pipeline". Foundation for Innovative New Diagnostics. 2020. https://www.finddx.org/covid-19/pipeline/.
- ↑ 75.0 75.1 "Epidemic response group ups coronavirus vaccine funding to $23.7 million". Reuters. 10 March 2020. https://www.reuters.com/article/us-health-coronavirus-vaccines-cepi/epidemic-response-group-ups-coronavirus-vaccine-funding-to-23-7-million-idUSKBN20X1PO.
- ↑ "CEPI's response to COVID-19". Coalition for Epidemic Preparedness Innovation, Oslo, Norway. 1 March 2020. https://cepi.net/covid-19/.
- ↑ "COVID-19 Therapeutics Accelerator: Bill & Melinda Gates Foundation, Wellcome, and Mastercard Launch Initiative to Speed Development and Access to Therapies for COVID-19". Bill and Melinda Gates Foundation. 10 March 2020. https://www.gatesfoundation.org/Media-Center/Press-Releases/2020/03/COVID-19-Therapeutics-Accelerator.
- ↑ "CEPI welcomes UK Government's funding and highlights need for $2 billion to develop a vaccine against COVID-19". Coalition for Epidemic Preparedness Innovations, Oslo, Norway. 6 March 2020. https://cepi.net/news_cepi/2-billion-required-to-develop-a-vaccine-against-the-covid-19-virus/.
- ↑ "Government of Canada funds 49 additional COVID-19 research projects – Details of the funded projects". Government of Canada. 23 March 2020. https://www.canada.ca/en/institutes-health-research/news/2020/03/government-of-canada-funds-49-additional-covid-19-research-projects-details-of-the-funded-projects.html.
- ↑ 80.0 80.1 "Canada to spend $192M on developing COVID-19 vaccine". Global News. 23 March 2020. https://globalnews.ca/news/6717883/coronavirus-canada-vaccine-spending/.
- ↑ "Vaccine watch: These are the efforts being made around the world". CTV News. 31 March 2020. https://www.ctvnews.ca/health/coronavirus/vaccine-watch-these-are-the-efforts-being-made-around-the-world-1.4875920.
- ↑ "Gates Foundation Expands Commitment to COVID-19 Response, Calls for International Collaboration". Bill & Melinda Gates Foundation. 15 April 2020. https://www.gatesfoundation.org/Media-Center/Press-Releases/2020/04/Gates-Foundation-Expands-Commitment-to-COVID-19-Response-Calls-for-International-Collaboration.
- ↑ 83.0 83.1 "Sixteen supercomputers tackle coronavirus cures in the US". CNET (ViacomCBS). 23 March 2020. https://www.cnet.com/news/sixteen-supercomputers-tackle-coronavirus-cures-in-us/.
- ↑ 84.0 84.1 "The COVID-19 High Performance Computing Consortium". The COVID-19 High Performance Computing Consortium. 2020. https://covid19-hpc-consortium.org/.
- ↑ Wiggers, Kyle (28 May 2020). "COVID-19 HPC Consortium pours 437 petaflops of compute power toward virus research". VentureBeat. https://venturebeat.com/2020/05/28/covid-19-hpc-consortium-pours-437-petaflops-of-compute-power-toward-virus-research/.
- ↑ "C3.ai, Microsoft, and Leading Universities Launch C3.ai Digital Transformation Institute". 26 March 2020. https://c3.ai/c3-ai-microsoft-and-leading-universities-launch-c3-ai-digital-transformation-institute/.
- ↑ "A.I. Versus the Coronavirus". The New York Times. 26 March 2020. https://www.nytimes.com/2020/03/26/science/ai-versus-the-coronavirus.html.
- ↑ "Help Cure Coronavirus with Your PC's Leftover Processing Power". 3 March 2020. https://www.tomshardware.com/news/folding-fight-coronavirus.
- ↑ "Folding@home takes up the fight against COVID-19 / 2019-nCoV". 27 February 2020. https://foldingathome.org/2020/02/27/foldinghome-takes-up-the-fight-against-covid-19-2019-ncov/.
- ↑ "Folding@home Turns Its Massive Crowdsourced Computer Network Against COVID-19". 16 March 2020. https://www.hpcwire.com/2020/03/16/foldinghome-turns-its-massive-crowdsourced-computer-network-against-covid-19/.
- ↑ Masterson, Victoria (22 December 2020). "Your computer can help scientists find a cure for COVID-19. Here's how". World Economic Forum. https://www.weforum.org/agenda/2020/12/citizen-scientists-crowdsourcing-covid-19-cure/.
- ↑ "Together We Are Powerful". https://foldingathome.org/?lng=en-US.
- ↑ von Delft, Frank; Calmiano, Mark; Chodera, John; Griffen, Ed; Lee, Alpha; London, Nir; Matviuk, Tatiana; Perry, Ben et al. (June 2021). "A white-knuckle ride of open COVID drug discovery" (in en). Nature 594 (7863): 330–332. doi:10.1038/d41586-021-01571-1. PMID 34127864. Bibcode: 2021Natur.594..330V.
- ↑ "Rosetta@home Rallies a Legion of Computers Against the Coronavirus" (in en-US). 24 March 2020. https://www.hpcwire.com/2020/03/24/rosettahome-rallies-a-legion-of-computers-against-the-coronavirus/.
- ↑ "OpenPandemics – COVID-19". IBM. 2020. https://www.worldcommunitygrid.org/research/opn1/overview.do.
- ↑ "'Solidarity' clinical trial for COVID-19 treatment". World Health Organization. 27 April 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments.
- ↑ 97.0 97.1 "WHO Director-General's opening remarks at the media briefing on COVID-19". World Health Organization. 25 May 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---25-may-2020.
- ↑ "WHO pauses hydroxychloroquine coronavirus trial over safety concerns". Global News (The Associated Press). 25 May 2020. https://globalnews.ca/news/6983283/who-hydroxychloroquine-trial/.
- ↑ "WHO restarts HCQ trial after Lancet concern over paper that trashed it". The Indian Express. 4 June 2020. https://indianexpress.com/article/coronavirus/who-hydroxychloroquine-trail-restart-lancet-6441507/. "When contacted, Soumya Swaminathan, chief scientist at WHO, told The Indian Express: Our data safety monitoring board reviewed the mortality data in Solidarity... They did not have concerns related to mortality between HCQ and standard of care. Hence, we have decided to resume the trial."
- ↑ 100.0 100.1 "WHO to launch multinational trial to jumpstart search for coronavirus drugs". STAT. 18 March 2020. https://www.statnews.com/2020/03/18/who-to-launch-multinational-trial-to-jumpstart-search-for-coronavirus-drugs/.
- ↑ "Adaptive Designs for Clinical Trials of Drugs and Biologics: Guidance for Industry" (PDF). 1 November 2019. https://www.fda.gov/media/78495/download.
- ↑ 102.0 102.1 "Adaptive designs in clinical trials: why use them, and how to run and report them". BMC Medicine 16 (1): 29. February 2018. doi:10.1186/s12916-018-1017-7. PMID 29490655.
- ↑ "WHO beginning Covid-19 therapy trial". Technology News: Science and Enterprise. 19 March 2020. https://sciencebusiness.technewslit.com/?p=38676.
- ↑ "Practical characteristics of adaptive design in Phase 2 and 3 clinical trials". Journal of Clinical Pharmacy and Therapeutics 43 (2): 170–180. April 2018. doi:10.1111/jcpt.12617. PMID 28850685.
- ↑ 105.0 105.1 "RECOVERY Trial". Nuffield Department of Population Health. 3 April 2020. https://www.recoverytrial.net/.
- ↑ Walsh, Fergus (20 June 2020). "At last some good news about coronavirus". https://www.bbc.co.uk/news/health-53096736.
- ↑ "RECOVERY trial rolled out across the UK". Nuffield Department of Population Health. 3 April 2020. https://www.recoverytrial.net/news/update.
- ↑ "Coronavirus: world's biggest trial of drug to treat Covid-19 begins in UK" (in en-GB). The Guardian. 17 April 2020. ISSN 0261-3077. https://www.theguardian.com/world/2020/apr/17/world-biggest-drug-trial-covid-19-uk.
- ↑ "Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19". 16 June 2020. https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_v2final.pdf.
- ↑ "Coronavirus: Dexamethasone proves first life-saving drug". BBC News Online. 16 June 2020. https://www.bbc.com/news/health-53061281.
- ↑ "Dexamethasone reduces death in hospitalised patients with severe respiratory complications of COVID-19". 16 June 2020. http://www.ox.ac.uk/news/2020-06-16-dexamethasone-reduces-death-hospitalised-patients-severe-respiratory-complications.
- ↑ "No clinical benefit from use of lopinavir-ritonavir in hospitalised COVID-19 patients studied in RECOVERY". RECOVERY Trial: Statement from the Chief Investigators. 29 June 2020. https://www.recoverytrial.net/files/lopinavir-ritonavir-recovery-statement-29062020_final.pdf.
- ↑ "Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial". Lancet 396 (10259): 1345–1352. October 2020. doi:10.1016/S0140-6736(20)32013-4. PMID 33031764.
- ↑ 114.0 114.1 "NIHR funds community COVID-19 antiviral trial" (in EN). https://www.nihr.ac.uk/news/nihr-funds-community-covid-19-antiviral-trial/29048.
- ↑ "Thousands needed to try a new Covid antiviral treatment" (in en-GB). BBC News. 25 January 2022. https://www.bbc.com/news/health-60117313.
- ↑ Pinching, John (22 February 2022). "PANORAMIC view of World's largest COVID study". https://www.pharmatimes.com/news/panoramic_view_of_worlds_largest_covid_study_1388052.
Further reading
- "Antibodies to watch in 2021". mAbs 13 (1): 1860476. 2021. doi:10.1080/19420862.2020.1860476. PMID 33459118.
- "Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options". Open Forum Infectious Diseases 7 (4): ofaa105. April 2020. doi:10.1093/ofid/ofaa105. PMID 32284951.
- "Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline". mAbs 12 (1): 1854149. 2020. doi:10.1080/19420862.2020.1854149. PMID 33319649.
- "COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19". Antib Ther 3 (3): 205–12. July 2020. doi:10.1093/abt/tbaa020. PMID 33215063.
External links
- R&D Blueprint and COVID-19, World Health Organization
- Coronaviruses by US National Institute for Allergy and Infectious Diseases
- COVID-19 therapeutics tracker Regulatory Affairs Professionals Society
- "COVID-19 treatments: research and development". European Medicines Agency (EMA). 18 February 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-covid-19/covid-19-treatments-research-development.
 |