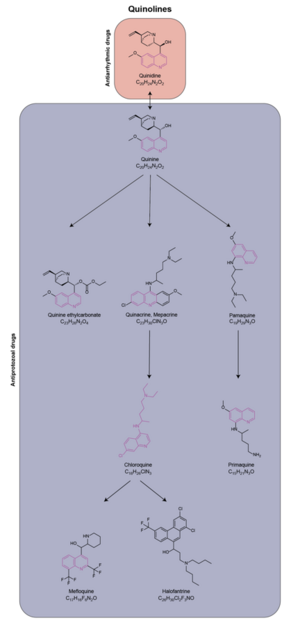
Chemistry:Chloroquine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈklɔːrəkwiːn/ |
| Trade names | Aralen, other |
| Other names | Chloroquine phosphate |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 1-2 months |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
| Formula | C18H26ClN3 |
| Molar mass | 319.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Chloroquine is a medication primarily used to prevent and treat malaria in areas where malaria remains sensitive to its effects.[1] Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication.[1] Chloroquine is also occasionally used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus.[1] While it has not been formally studied in pregnancy, it appears safe.[1][2] It was studied to treat COVID-19 early in the pandemic, but these studies were largely halted in the summer of 2020, and the NIH does not recommend its use for this purpose.[3] It is taken by mouth.[1]
Common side effects include muscle problems, loss of appetite, diarrhea, and skin rash.[1] Serious side effects include problems with vision, muscle damage, seizures, and low blood cell levels.[1][4] Chloroquine is a member of the drug class 4-aminoquinoline.[1] As an antimalarial, it works against the asexual form of the malaria parasite in the stage of its life cycle within the red blood cell.[1] How it works in rheumatoid arthritis and lupus erythematosus is unclear.[1]
Chloroquine was discovered in 1934 by Hans Andersag.[5][6] It is on the World Health Organization's List of Essential Medicines.[7] It is available as a generic medication.[1]
Medical uses
Malaria
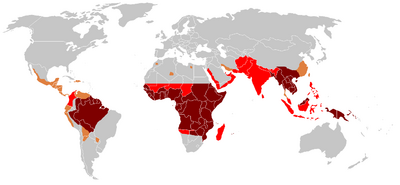
♦ Elevated occurrence of chloroquine- or multi-resistant malaria
♦ Occurrence of chloroquine-resistant malaria
♦ No Plasmodium falciparum or chloroquine-resistance
♦ No malaria
Chloroquine has been used in the treatment and prevention of malaria from Plasmodium vivax, P. ovale, and P. malariae. It is generally not used for Plasmodium falciparum as there is widespread resistance to it.[9][10]
Chloroquine has been extensively used in mass drug administrations, which may have contributed to the emergence and spread of resistance. It is recommended to check if chloroquine is still effective in the region prior to using it.[11] In areas where resistance is present, other antimalarials, such as mefloquine or atovaquone, may be used instead. The Centers for Disease Control and Prevention recommend against treatment of malaria with chloroquine alone due to more effective combinations.[12]
Amebiasis
In treatment of amoebic liver abscess, chloroquine may be used instead of or in addition to other medications in the event of failure of improvement with metronidazole or another nitroimidazole within five days or intolerance to metronidazole or a nitroimidazole.[13]
Rheumatic disease
As it mildly suppresses the immune system, chloroquine is used in some autoimmune disorders, such as rheumatoid arthritis and has an off label indication for lupus erythematosus.[1]
Side effects
Side effects include blurred vision, nausea, vomiting, abdominal cramps, headache, diarrhea, swelling legs/ankles, shortness of breath, pale lips/nails/skin, muscle weakness, easy bruising/bleeding, hearing and mental problems.[14][15]
- Unwanted/uncontrolled movements (including tongue and face twitching)[14]
- Deafness or tinnitus.[14]
- Nausea, vomiting, diarrhea, abdominal cramps.[15]
- Headache.[14]
- Mental/mood changes (such as confusion, personality changes, unusual thoughts/behavior, depression, feeling being watched, hallucinating)[14][15]
- Signs of serious infection (such as high fever, severe chills, persistent sore throat)[14]
- Skin itchiness, skin color changes, hair loss, and skin rashes.[15][16]
- Chloroquine-induced itching is very common among black Africans (70%), but much less common in other races. It increases with age, and is so severe as to stop compliance with drug therapy. It is increased during malaria fever; its severity is correlated to the malaria parasite load in blood. Some evidence indicates it has a genetic basis and is related to chloroquine action with opiate receptors centrally or peripherally.[17]
- Unpleasant metallic taste
- This could be avoided by "taste-masked and controlled release" formulations such as multiple emulsions.[18]
- Chloroquine retinopathy
- Electrocardiographic changes[19]
- This manifests itself as either conduction disturbances (bundle-branch block, atrioventricular block) or Cardiomyopathy – often with hypertrophy, restrictive physiology, and congestive heart failure. The changes may be irreversible. Only two cases have been reported requiring heart transplantation, suggesting this particular risk is very low. Electron microscopy of cardiac biopsies show pathognomonic cytoplasmic inclusion bodies.
- Pancytopenia, aplastic anemia, reversible agranulocytosis, low blood platelets, neutropenia.[20]
Pregnancy
Chloroquine has not been shown to have any harmful effects on the fetus when used in the recommended doses for malarial prophylaxis.[21] Small amounts of chloroquine are excreted in the breast milk of lactating women. However, this drug can be safely prescribed to infants, the effects are not harmful. Studies with mice show that radioactively tagged chloroquine passed through the placenta rapidly and accumulated in the fetal eyes which remained present five months after the drug was cleared from the rest of the body.[20][22] Women who are pregnant or planning on getting pregnant are still advised against traveling to malaria-risk regions.[21]
Elderly
There is not enough evidence to determine whether chloroquine is safe to be given to people aged 65 and older. Since it is cleared by the kidneys, toxicity should be monitored carefully in people with poor kidney functions.[20]
Drug interactions
Chloroquine has a number of drug–drug interactions that might be of clinical concern:[citation needed]
- Ampicillin- levels may be reduced by chloroquine;[20]
- Antacids- may reduce absorption of chloroquine;[20]
- Cimetidine- may inhibit metabolism of chloroquine; increasing levels of chloroquine in the body;[20]
- Cyclosporine- levels may be increased by chloroquine;[20] and
- Mefloquine- may increase risk of convulsions.[20]
Overdose
Chloroquine, in overdose, has a risk of death of about 20%.[23] It is rapidly absorbed from the gut with an onset of symptoms generally within an hour.[24] Symptoms of overdose may include sleepiness, vision changes, seizures, stopping of breathing, and heart problems such as ventricular fibrillation and low blood pressure.[23][24] Low blood potassium may also occur.[23]
While the usual dose of chloroquine used in treatment is 10 mg/kg, toxicity begins to occur at 20 mg/kg, and death may occur at 30 mg/kg.[23] In children as little as a single tablet can cause problems.[24]
Treatment recommendations include early mechanical ventilation, cardiac monitoring, and activated charcoal.[23] Intravenous fluids and vasopressors may be required with epinephrine being the vasopressor of choice.[23] Seizures may be treated with benzodiazepines.[23] Intravenous potassium chloride may be required, however this may result in high blood potassium later in the course of the disease.[23] Dialysis has not been found to be useful.[23]
Pharmacology
Absorption of chloroquine is rapid and primarily happens in the gastrointestinal tract.[25] It is widely distributed in body tissues.[26] Protein binding in plasma ranges from 46% to 79%.[27] Its metabolism is partially hepatic, giving rise to its main metabolite, desethylchloroquine.[28] Its excretion is ≥50% as unchanged drug in urine, where acidification of urine increases its elimination.[citation needed] It has a very high volume of distribution, as it diffuses into the body's adipose tissue.[citation needed]
Accumulation of the drug may result in deposits that can lead to blurred vision and blindness.[29] It and related quinines have been associated with cases of retinal toxicity, particularly when provided at higher doses for longer times.[citation needed] With long-term doses, routine visits to an ophthalmologist are recommended.[citation needed]
Chloroquine is also a lysosomotropic agent, meaning it accumulates preferentially in the lysosomes of cells in the body.[citation needed] The pKa for the quinoline nitrogen of chloroquine is 8.5, meaning it is about 10% deprotonated at physiological pH (per the Henderson-Hasselbalch equation).[citation needed] This decreases to about 0.2% at a lysosomal pH of 4.6.[citation needed] Because the deprotonated form is more membrane-permeable than the protonated form, a quantitative "trapping" of the compound in lysosomes results.[citation needed]
Mechanism of action
Malaria

The lysosomotropic character of chloroquine is believed to account for much of its antimalarial activity; the drug concentrates in the acidic food vacuole of the parasite and interferes with essential processes. Its lysosomotropic properties further allow for its use for in vitro experiments pertaining to intracellular lipid related diseases,[30][31] autophagy, and apoptosis.[32]
Inside red blood cells, the malarial parasite, which is then in its asexual lifecycle stage, must degrade hemoglobin to acquire essential amino acids, which the parasite requires to construct its own protein and for energy metabolism. Digestion is carried out in a vacuole of the parasitic cell.[citation needed]
Hemoglobin is composed of a protein unit (digested by the parasite) and a heme unit (not used by the parasite). During this process, the parasite releases the toxic and soluble molecule heme. The heme moiety consists of a porphyrin ring called Fe(II)-protoporphyrin IX (FP). To avoid destruction by this molecule, the parasite biocrystallizes heme to form hemozoin, a nontoxic molecule. Hemozoin collects in the digestive vacuole as insoluble crystals.[citation needed]
Chloroquine enters the red blood cell by simple diffusion, inhibiting the parasite cell and digestive vacuole. Chloroquine (CQ) then becomes protonated (to CQ2+), as the digestive vacuole is known to be acidic (pH 4.7); chloroquine then cannot leave by diffusion. Chloroquine caps hemozoin molecules to prevent further biocrystallization of heme, thus leading to heme buildup. Chloroquine binds to heme (or FP) to form the FP-chloroquine complex; this complex is highly toxic to the cell and disrupts membrane function. Action of the toxic FP-chloroquine and FP results in cell lysis and ultimately parasite cell autodigestion.[33] Parasites that do not form hemozoin are therefore resistant to chloroquine.[34]
Resistance in malaria
Since the first documentation of P. falciparum chloroquine resistance in the 1950s, resistant strains have appeared throughout East and West Africa, Southeast Asia, and South America. The effectiveness of chloroquine against P. falciparum has declined as resistant strains of the parasite evolved.
Resistant parasites are able to rapidly remove chloroquine from the digestive vacuole using a transmembrane pump. Chloroquine-resistant parasites pump chloroquine out at 40 times the rate of chloroquine-sensitive parasites; the pump is coded by the P. falciparum chloroquine resistance transporter (PfCRT) gene.[35] The natural function of the chloroquine pump is to transport peptides: mutations to the pump that allow it to pump chloroquine out impairs its function as a peptide pump and comes at a cost to the parasite, making it less fit.[36]
Resistant parasites also frequently have mutation in the ABC transporter P. falciparum multidrug resistance (PfMDR1) gene, although these mutations are thought to be of secondary importance compared to PfCRT. An altered chloroquine-transporter protein, CG2 has been associated with chloroquine resistance, but other mechanisms of resistance also appear to be involved.[37]
Verapamil, a Ca2+ channel blocker, has been found to restore both the chloroquine concentration ability and sensitivity to this drug. Other agents which have been shown to reverse chloroquine resistance in malaria are chlorpheniramine, gefitinib, imatinib, tariquidar and zosuquidar.[38]
As of 2014[update] chloroquine is still effective against poultry malaria in Thailand. Sohsuebngarm et al. 2014 test P. gallinaceum at Chulalongkorn University and find the parasite is not resistant.[39]: 1237 Sertraline, fluoxetine and paroxetine reverse chloroquine resistance, making resistant biotypes susceptible if used in a cotreatment.[40]
Antiviral
Chloroquine has antiviral effects against some viruses.[41] It increases late endosomal and lysosomal pH, resulting in impaired release of the virus from the endosome or lysosome – release of the virus requires a low pH. The virus is therefore unable to release its genetic material into the cell and replicate.[42][43]
Chloroquine also seems to act as a zinc ionophore that allows extracellular zinc to enter the cell and inhibit viral RNA-dependent RNA polymerase.[44][45]
Other
Chloroquine inhibits thiamine uptake.[46] It acts specifically on the transporter SLC19A3.
Against rheumatoid arthritis, it operates by inhibiting lymphocyte proliferation, phospholipase A2, antigen presentation in dendritic cells, release of enzymes from lysosomes, release of reactive oxygen species from macrophages, and production of IL-1.
History
In Peru, the indigenous people extracted the bark of the Cinchona tree (Cinchona officinalis)[47] and used the extract to fight chills and fever in the seventeenth century. In 1633, this herbal medicine was introduced in Europe, where it was given the same use and also began to be used against malaria. The quinoline antimalarial drug quinine was isolated from the extract in 1820.[48]: 130–131
After World War I, the German government sought alternatives to quinine. Chloroquine, a synthetic analogue with the same mechanism of action was discovered in 1934, by Hans Andersag and coworkers at the Bayer laboratories, who named it Resochin.[49][50] It was ignored for a decade, because it was considered too toxic for human use. Instead, in World War II, the German Africa Corps used the chloroquine analogue 3-methyl-chloroquine, known as Sontochin. After Allied forces arrived in Tunis, Sontochin fell into the hands of Americans, who sent the material back to the United States for analysis, leading to renewed interest in chloroquine.[51][52] United States government-sponsored clinical trials for antimalarial drug development showed unequivocally that chloroquine has a significant therapeutic value as an antimalarial drug.[48]: 61–66 It was introduced into clinical practice in 1947 for the prophylactic treatment of malaria.[53]
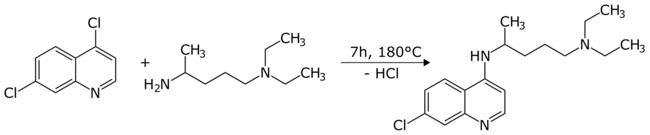
Chemical synthesis
The first synthesis of chloroquine was disclosed in a patent filed by IG Farben in 1937.[54] In the final step, 4,7-dichloroquinoline was reacted with 1-diethylamino-4-aminopentane.
By 1949, chloroquine manufacturing processes had been established to allow its widespread use.[55]
Society and culture

Formulations
Chloroquine comes in tablet form as the phosphate, sulfate, and hydrochloride salts. Chloroquine is usually dispensed as the phosphate.[56]
Names
Brand names include Chloroquine FNA, Resochin, Dawaquin, and Lariago.[57]
Other animals
Chloroquine, in various chemical forms, is used to treat and control surface growth of anemones and algae, and many protozoan infections in aquariums,[58] e.g. the fish parasite Amyloodinium ocellatum.[59] It is also used in poultry malaria.[39]: 1237
Research
Chloroquine was proposed as a treatment for SARS, with in vitro tests inhibiting the severe acute respiratory syndrome coronavirus (SARS-CoV).[60][61] In October 2004, a published report stated that chloroquine acts as an effective inhibitor of the replication of SARS-CoV in vitro.[60] In August 2005, a peer-reviewed study confirmed and expanded upon the results.[62]
Chloroquine was being considered in 2003, in pre-clinical models as a potential agent against chikungunya fever.[63]
COVID-19
Other
The radiosensitizing and chemosensitizing properties of chloroquine are being evaluated for anticancer strategies in humans.[64][65] In biomedicinal science, chloroquine is used for in vitro experiments to inhibit lysosomal degradation of protein products. Chloroquine and its modified forms have also been evaluated as treatment options for inflammatory conditions like rheumatoid arthritis and inflammatory bowel disease.[66]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Aralen Phosphate". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/chloroquine-phosphate.html.
- ↑ "Chloroquine Use During Pregnancy". https://www.drugs.com/pregnancy/chloroquine.html. "There are no controlled data in human pregnancies."
- ↑ "Chloroquine or Hydroxychloroquine" (in en). National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/chloroquine-or-hydroxychloroquine-with-or-without-azithromycin/.
- ↑ "Chapter 89 – Drug Toxicity of the Posterior Segment" (in en). Retina (Fifth ed.). W.B. Saunders. 2013. pp. 1532–1554. doi:10.1016/B978-1-4557-0737-9.00089-8. ISBN 978-1-4557-0737-9. https://entokey.com/drug-toxicity-of-the-posterior-segment/. Retrieved 25 March 2020.
- ↑ Manson's tropical diseases. (22nd ed.). [Edinburgh]: Saunders. 2009. p. 1240. ISBN 978-1-4160-4470-3. https://books.google.com/books?id=CF2INI0O6l0C&pg=PA1240. Retrieved 9 September 2017.
- ↑ Chemistry of Antibiotics and Related Drugs. Springer. 2016. p. 184. ISBN 978-3-319-40746-3. https://books.google.com/books?id=vgXWDAAAQBAJ&pg=PA184. Retrieved 9 September 2017.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06.
- ↑ "Frequently Asked Questions (FAQs): If I get malaria, will I have it for the rest of my life?". US Centers for Disease Control and Prevention. 8 February 2010. https://www.cdc.gov/malaria/about/faqs.html#treatment.
- ↑ "Antimalarial drug resistance in Africa: strategies for monitoring and deterrence". Malaria: Drugs, Disease and Post-genomic Biology. Current Topics in Microbiology and Immunology. 295. Springer. 2005. pp. 55–79. doi:10.1007/3-540-29088-5_3. ISBN 3-540-25363-7. https://archive.org/details/malariadrugsdise0000unse/page/55.
- ↑ "Antimalarial Multi-Drug Resistance in Asia: Mechanisms and Assessment". Malaria: Drugs, Disease and Post-genomic Biology. Current Topics in Microbiology and Immunology. 295. Springer. 2005. pp. 39–53. doi:10.1007/3-540-29088-5_2. ISBN 978-3-540-25363-1.
- ↑ "Chloroquine phosphate tablet – chloroquine phosphate tablet, coated". http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9b585ad5-ae86-4403-b83f-8d8363d43da5.
- ↑ CDC. Health information for international travel 2001–2002. Atlanta, Georgia: U.S. Department of Health and Human Services, Public Health Service, 2001.
- ↑ Amebic Hepatic Abscesses~treatment at eMedicine
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 "Drugs & Medications". https://www.webmd.com/drugs/2/drug-8633/chloroquine-oral/details.
- ↑ 15.0 15.1 15.2 15.3 "Chloroquine Side Effects: Common, Severe, Long Term". https://www.drugs.com/sfx/chloroquine-side-effects.html.
- ↑ "Chloroquine: MedlinePlus Drug Information". https://medlineplus.gov/druginfo/meds/a682318.html.
- ↑ "Mechanisms of chloroquine-induced pruritus". Clinical Pharmacology and Therapeutics 68 (3): 336. September 2000. PMID 11014416.
- ↑ "Slow release of chloroquine phosphate from multiple taste-masked W/O/W multiple emulsions". Journal of Microencapsulation 11 (6): 641–648. 1994. doi:10.3109/02652049409051114. PMID 7884629.
- ↑ "Chloroquine cardiomyopathy - a review of the literature". Immunopharmacology and Immunotoxicology 35 (3): 434–442. June 2013. doi:10.3109/08923973.2013.780078. PMID 23635029.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 "Chloroquine phosphate tablet". 8 October 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee944d28-f596-4163-a502-e779c0d622bc.
- ↑ 21.0 21.1 "Malaria – Chapter 3 – 2016 Yellow Book". http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/malaria.
- ↑ "Accumulation of chorio-retinotoxic drugs in the foetal eye". Nature 227 (5264): 1257–1258. September 1970. doi:10.1038/2271257a0. PMID 5452818. Bibcode: 1970Natur.227.1257U.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 "Hydroxychloroquine overdose: case report and recommendations for management". European Journal of Emergency Medicine 15 (1): 16–18. February 2008. doi:10.1097/MEJ.0b013e3280adcb56. PMID 18180661.
- ↑ 24.0 24.1 24.2 "Are 1-2 dangerous? Chloroquine and hydroxychloroquine exposure in toddlers". The Journal of Emergency Medicine 28 (4): 437–443. May 2005. doi:10.1016/j.jemermed.2004.12.011. PMID 15837026.
- ↑ "Chloroquine". §6.1 Absorption by route of exposure. http://www.inchem.org/documents/pims/pharm/chloroqu.htm#SectionTitle:6.1%20Absorption%20by%20route%20of%20exposure.
- ↑ "Tissue and blood concentrations of chloroquine following chronic administration in the rat". The Journal of Pharmacy and Pharmacology 34 (11): 733–735. November 1982. doi:10.1111/j.2042-7158.1982.tb06211.x. PMID 6129306.
- ↑ "Characterization of chloroquine plasma protein binding in man". British Journal of Clinical Pharmacology 15 (3): 375–377. March 1983. doi:10.1111/j.1365-2125.1983.tb01513.x. PMID 6849768.
- ↑ "In vitro metabolism of chloroquine: identification of CYP2C8, CYP3A4, and CYP2D6 as the main isoforms catalyzing N-desethylchloroquine formation". Drug Metabolism and Disposition 31 (6): 748–754. June 2003. doi:10.1124/dmd.31.6.748. PMID 12756207.
- ↑ "[Visual loss under chloroquine treatment-and not (only) due to bull's eye maculopathy!]" (in German). Der Ophthalmologe 118 (9): 953–955. September 2021. doi:10.1007/s00347-020-01288-y. PMID 33300096.
- ↑ "Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation: possible implications of lysosomal dysfunction in macular degeneration". Cell & Bioscience 1 (1): 10. March 2011. doi:10.1186/2045-3701-1-10. PMID 21711726.
- ↑ "Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61". The Journal of Neuroscience 30 (17): 5948–5957. April 2010. doi:10.1523/JNEUROSCI.0157-10.2010. PMID 20427654.
- ↑ "Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells". Neuro-Oncology 12 (4): 389–400. April 2010. doi:10.1093/neuonc/nop046. PMID 20308316.
- ↑ "Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors". Parasitology Research 100 (4): 671–676. March 2007. doi:10.1007/s00436-006-0313-x. PMID 17111179.
- ↑ "Replication of Plasmodium in reticulocytes can occur without hemozoin formation, resulting in chloroquine resistance". The Journal of Experimental Medicine 212 (6): 893–903. June 2015. doi:10.1084/jem.20141731. PMID 25941254. PMC 4451122. https://lirias.kuleuven.be/bitstream/123456789/500975/3/2015113.pdf. Retrieved 4 November 2018.
- ↑ "Chloroquine transport via the malaria parasite's chloroquine resistance transporter". Science 325 (5948): 1680–1682. September 2009. doi:10.1126/science.1175667. PMID 19779197. Bibcode: 2009Sci...325.1680M.
- ↑ "The natural function of the malaria parasite's chloroquine resistance transporter". Nature Communications 11 (1): 3922. August 2020. doi:10.1038/s41467-020-17781-6. PMID 32764664. Bibcode: 2020NatCo..11.3922S.
- ↑ Essentials of Medical Pharmacology (fifth ed.). Jaypee Brothers Medical Publisher Ltd. 2003. pp. 739–740.
- ↑ "Chemosensitization potential of P-glycoprotein inhibitors in malaria parasites". Experimental Parasitology 134 (2): 235–243. June 2013. doi:10.1016/j.exppara.2013.03.022. PMID 23541983.
- ↑ 39.0 39.1 "Protozoal Infections". Diseases of Poultry (14 ed.). Wiley. 22 November 2019. ISBN 9781119371199.
- ↑ "Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors". International Journal of Antimicrobial Agents (International Society of Chemotherapy (Elsevier)) 14 (3): 177–180. April 2000. doi:10.1016/s0924-8579(99)00154-5. PMID 10773485.
- ↑ "Effects of chloroquine on viral infections: an old drug against today's diseases?". The Lancet. Infectious Diseases 3 (11): 722–727. November 2003. doi:10.1016/s1473-3099(03)00806-5. PMID 14592603.
- ↑ "Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases". Pharmacology Research & Perspectives 5 (1): e00293. February 2017. doi:10.1002/prp2.293. PMID 28596841.
- ↑ "Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus". Journal of Virology 76 (22): 11440–11446. November 2002. doi:10.1128/JVI.76.22.11440-11446.2002. PMID 12388705.
- ↑ "Chloroquine is a zinc ionophore". PLOS ONE 9 (10): e109180. 1 October 2014. doi:10.1371/journal.pone.0109180. PMID 25271834. Bibcode: 2014PLoSO...9j9180X.
- ↑ "Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture". PLOS Pathogens 6 (11): e1001176. November 2010. doi:10.1371/journal.ppat.1001176. PMID 21079686.
- ↑ "Discovering thiamine transporters as targets of chloroquine using a novel functional genomics strategy". PLOS Genetics 8 (11): e1003083. 2012. doi:10.1371/journal.pgen.1003083. PMID 23209439.
- ↑ "Cinchona officinalis – L.". 2010–2020. https://pfaf.org/user/Plant.aspx?LatinName=Cinchona+officinalis.
- ↑ 48.0 48.1 Institute of Medicine (US) Committee on the Economics of Antimalarial Drug (2004). Saving lives, buying time : economics of malaria drugs in an age of resistance. National Academies Press. doi:10.17226/11017. ISBN 9780309092180.
- ↑ "Antimalarials: construction of molecular hybrids based on chloroquine". Universitas Scientiarum 13 (3): 306–320. September 2008. http://www.scielo.org.co/scielo.php?pid=S0122-74832008000300010&script=sci_arttext.
- ↑ "From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy". Parasitology Research 111 (1): 1–6. July 2012. doi:10.1007/s00436-012-2886-x. PMID 22411634.
- ↑ Drug Discovery. A History.. Wiley. 2005. ISBN 0471899801.
- ↑ "Sontochin as a guide to the development of drugs against chloroquine-resistant malaria". Antimicrobial Agents and Chemotherapy 56 (7): 3475–3480. July 2012. doi:10.1128/AAC.00100-12. PMID 22508305.
- ↑ "The History of Malaria, an Ancient Disease". Centers for Disease Control. 29 July 2019. https://www.cdc.gov/malaria/history/index.htm#chloroquine.
- ↑ ; Breitner, Stefan & Jung, Heinrich"Process for the preparation of quinoline compounds containing amino groups with basic substituents in the 4-position" DE patent 683692, issued 1939-11-13, assigned to IG Farbenindustrie AG
- ↑ "Chloroquine manufacture". Industrial & Engineering Chemistry 41 (4): 654–662. 1 April 1949. doi:10.1021/ie50472a002.
- ↑ "Chloroquine". National Institutes of Health. https://pubchem.ncbi.nlm.nih.gov/compound/chloroquine#section=U-S-Imports.
- ↑ "Ipca Laboratories: Formulations – Branded". https://www.ipca.com/pharmaceutical-formulations-manufacturers-india.html.
- ↑ "Aquarium Fish: Chloroquine: A "New" Drug for Treating Fish Diseases". 20 February 2013. https://reefs.com/magazine/aquarium-fish-chloroquine-a-new-drug-for-treating-fish-diseases/.
- ↑ "Amyloodinium ocellatum, an Important Parasite of Cultured Marine Fish". http://agrilife.org/fisheries/files/2013/09/SRAC-Publication-No.-4705-Amyloodinium-ocellatum-an-Important-Parasite-of-Cultured-Marine-Fish.pdf.
- ↑ 60.0 60.1 "In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine". Biochemical and Biophysical Research Communications 323 (1): 264–268. October 2004. doi:10.1016/j.bbrc.2004.08.085. PMID 15351731.
- ↑ "New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?". International Journal of Antimicrobial Agents 55 (5): 105938. May 2020. doi:10.1016/j.ijantimicag.2020.105938. PMID 32171740.
- ↑ "Chloroquine is a potent inhibitor of SARS coronavirus infection and spread". Virology Journal 2: 69. August 2005. doi:10.1186/1743-422X-2-69. PMID 16115318.
- ↑ "Effects of chloroquine on viral infections: an old drug against today's diseases?". The Lancet. Infectious Diseases 3 (11): 722–727. November 2003. doi:10.1016/S1473-3099(03)00806-5. PMID 14592603.
- ↑ "Risks and benefits of chloroquine use in anticancer strategies". The Lancet. Oncology 7 (10): 792–793. October 2006. doi:10.1016/S1470-2045(06)70875-0. PMID 17012039.
- ↑ "Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial". Annals of Internal Medicine 144 (5): 337–343. March 2006. doi:10.7326/0003-4819-144-5-200603070-00008. PMID 16520474.
"Summaries for patients. Adding chloroquine to conventional chemotherapy and radiotherapy for glioblastoma multiforme". Annals of Internal Medicine 144 (5): I31. March 2006. doi:10.7326/0003-4819-144-5-200603070-00004. PMID 16520470. - ↑ "Chloroquine". StatPearls. Treasure Island (FL): StatPearls Publishing LLC.. January 2020. https://www.ncbi.nlm.nih.gov/books/NBK551512/.
External links
- "Medicines for the Prevention of Malaria While Traveling – Chloroquine (Aralen)". U.S. Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/malaria/resources/pdf/fsp/drugs/Chloroquine.pdf.
 |