Chemistry:Favipiravir
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Avigan (アビガン Abigan), Avifavir,[1] Areplivir,[2] others |
| Other names | T-705, favipira, favilavir |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
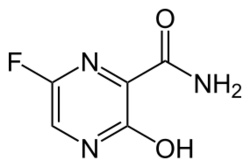
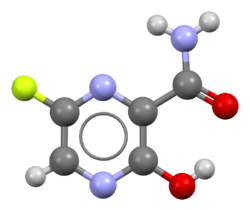
| Formula | C5H4FN3O2 |
| Molar mass | 157.104 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Favipiravir, sold under the brand name Avigan among others,[3] is an antiviral medication used to treat influenza in Japan.[4] It is also being studied to treat a number of other viral infections, including SARS-CoV-2.[4] Like the experimental antiviral drugs T-1105 and T-1106, it is a pyrazinecarboxamide derivative.[5]
It is being developed and manufactured by Toyama Chemical (a subsidiary of Fujifilm) and was approved for medical use in Japan in 2014.[6] In 2016, Fujifilm licensed it to Zhejiang Hisun Pharmaceutical Co.[7] It became a generic drug in 2019.[citation needed]
Medical use
Favipiravir has been approved to treat influenza in Japan.[6] It is, however, only indicated for novel influenza (strains that cause more severe disease) rather than seasonal influenza.[6][8] As of 2020, the probability of resistance developing appears low.[6]
Side effects
There is evidence that use during pregnancy may result in harm to the baby.[6] Teratogenic and embryotoxic effects were shown on four animal species.[6][9] In one case report, a 6-month old infant developed benign bright blue discolouration of the cornea after treatment with favipiravir which resolved after treatment cessation.[10]
Mechanism of action
The mechanism of its actions is thought to be related to the selective inhibition of viral RNA-dependent RNA polymerase.[11] Favipiravir is a prodrug that is metabolized to its active form, favipiravir-ribofuranosyl-5'-triphosphate (favipiravir-RTP), available in both oral and intravenous formulations.[12][13] In 2014, favipiravir was approved in Japan for stockpiling against influenza pandemics.[14] However, favipiravir has not been shown to be effective in primary human airway cells, casting doubt on its efficacy in influenza treatment.[15]
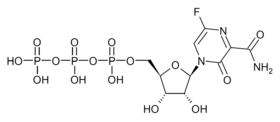
Favipiravir-RTP is a nucleoside analogue. It mimics both guanosine and adenosine for the viral RdRP. Incorporating two such bases in a row stops primer extension, although it is unclear how as of 2013.[11]
Society and culture
Legal status
The US Department of Defense developed favipiravir in partnership with MediVector, Inc. as a broad-spectrum antiviral and sponsored it through FDA Phase II and Phase III clinical trials, where it demonstrated safety in humans and efficacy against the influenza virus.[16] favipiravir remains unapproved in the UK and the USA.[17] In 2014, Japan approved favipiravir for treating influenza strains unresponsive to current antivirals.[18] Toyama Chemical initially hoped that favipiravir would become a new influenza medication that could replace oseltamivir (brand name Tamiflu). However, animal experiments show the potential for teratogenic effects, and the approval of production by The Ministry of Health, Labor and Welfare was greatly delayed and the production condition is limited only in an emergency in Japan.[19]
Despite limited data on efficacy, as of March 2021 favipiravir is widely prescribed for outpatient treatment of mild to moderate COVID-19 in Egypt,[20] Hungary[21] and Serbia.[22] Patients are required to sign a consent form before obtaining the drug.[citation needed]
Brand names

Favipiravir is sold under the brand names Avigan (アビガン Abigan), Avifavir,[1] Avipiravir,[23] Areplivir,[2] FabiFlu,[24] Favipira,[25] Reeqonus,[26][27] and Qifenda.
Use in Russia
Coronavir is the brand name of favipiravir used in Russia , where it is approved for the treatment of COVID-19. It is produced and sold by R-Pharm.[28][29] Coronavir was approved for use in Russia in hospitals in July 2020, and in September 2020 it received approval for prescription sales for outpatient use.[30]
Research
COVID-19
Favipiravir, as an antiviral drug, has been authorized for treating COVID-19 in several countries including Japan, Russia, Serbia, Turkey, India, and Thailand, under emergency provisions.[31][32][33][34] A rapid meta-review in September 2020 (analyzing four studies) noted that the drug led to clinical and radiological improvements; however, no reduction in mortality or differences in oxygen-support requirement were observed and more rigorous studies were sought.[35][36]
(As of May 2021), large-cohort clinical trials are underway.[37]
Ebola
Research in 2014, suggested that favipiravir may have efficacy against Ebola based on studies in mouse models; efficacy in humans was unaddressed.[38][39][40]
During the 2014 West Africa Ebola virus outbreak, a French nurse who contracted Ebola while volunteering for Médecins Sans Frontières (MSF) in Liberia reportedly recovered after receiving a course of favipiravir.[41] A clinical trial investigating the use of favipiravir against Ebola virus disease began in Guéckédou, Guinea, in December 2014.[42] Preliminary results presented in 2016 at the Conference on Retroviruses and Opportunistic Infections (CROI), later published, showed a decrease in mortality in patients with low-to-moderate levels of virus in blood, but no effect on patients with high levels (the group at a higher risk of death).[43][44][45] The trial design was concomitantly criticised for using only historical controls.[46]
Nipah
Nipah virus is a causative agent of outbreaks of encephalitis with pneumonia and has a high case fatality rate. The first outbreak occurred in Malaysia-Singapore, related to contact with pigs in slaughterhouses and an outbreak in Philippines related to slaughter of horses, most other outbreaks have affected India and Bangladesh. in Bangladesh outbreaks are often associated with consumption of raw date palm sap contaminated by saliva and urine of fruit bats.[47] In a study published in the Scientific Reports, Syrian hamster model for Nipah virus infection was used, which closely mirrors most aspects of human disease, such as widespread vasculitis, pneumonia, and encephalitis. The hamsters were infected with a lethal dose of 104 PFU NiV-M via the intraperitoneal (i.p.) route similar to previous studies and treatment was initiated immediately after infection. Favipiravir was administered twice daily via the peroral (p.o.) route for 14 days. The treated hamsters displayed 100% survival and no obvious morbidity after lethal NiV challenge, whereas all the control cases died of severe disease.[48]
Other
In experiments in animals favipiravir has shown activity against West Nile virus, yellow fever virus, foot-and-mouth disease virus as well as other flaviviruses, arenaviruses, bunyaviruses and alphaviruses.[49] Activity against enteroviruses[50] and Rift Valley fever virus has also been demonstrated.[51] Favipiravir has showed limited efficacy against Zika virus in animal studies, but was less effective than other antivirals such as MK-608.[52] The agent has also shown some efficacy against rabies,[53] and has been used experimentally in some humans infected with the virus.[54]
Tautomerism
The possible tautomerism of favipiravir has been investigated computationally[55] and experimentally.[56] It was found that the enol-like form was substantially more stable in organic solvents than the keto-like form, meaning that Favipiravir likely exists almost exclusively in the enol-like form. In aqueous solution the keto-like tautomer is substantially stabilized due to the specific interaction with the water molecules. Upon protonation the keto form is switched on.[citation needed]
-
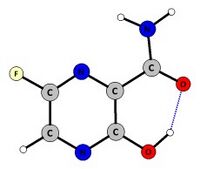
Enol-like tautomeric form
-
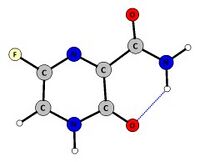
Keto-like tautomeric form
References
- ↑ 1.0 1.1 "Avifavir". Russian drug reference. Medum.ru. https://medum.ru/avifavir.
- ↑ 2.0 2.1 "Arelpivir". Russian drug reference. Medum.ru. https://medum.ru/areplivir.
- ↑ "Glenmark launches Covid-19 drug FabiFlu, priced at Rs 103 per tablet". Business Standard India. Press Trust of India. 20 June 2020. https://www.business-standard.com/article/companies/glenmark-launches-covid-19-drug-fabiflu-priced-at-rs-103-per-tablet-120062000872_1.html.
- ↑ 4.0 4.1 "Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection". Clinical Pharmacology and Therapeutics 108 (2): 242–247. August 2020. doi:10.1002/cpt.1844. PMID 32246834.
- ↑ "T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections". Antiviral Research 82 (3): 95–102. June 2009. doi:10.1016/j.antiviral.2009.02.198. PMID 19428599.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 "Favipiravir, an anti-influenza drug against life-threatening RNA virus infections". Pharmacology & Therapeutics 209: 107512. May 2020. doi:10.1016/j.pharmthera.2020.107512. PMID 32097670.
- ↑ EJ Lane (22 June 2016). "Fujifilm in Avigan API license with Zhejiang Hisun Pharmaceuticals". Fierce Pharma. https://www.fiercepharma.com/pharma-asia/japan-s-fujifilm-licenses-flu-drug-api-to-china-s-zhejiang-hisun-pharmaceutical-a-first.
- ↑ "Information of Avigan Tablet in relation to Covid-19". FUJIFILM Toyama Chemical Co., Ltd. https://www.fujifilm.com/fftc/en/avigan.
- ↑ "A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic?". Journal of Virus Eradication 6 (2): 45–51. April 2020. doi:10.1016/S2055-6640(20)30016-9. PMID 32405421.
- ↑ Jiravisitkul, Paveewan; Thonginnetra, Saraiorn; Wongvisavavit, Rintra (2023). "Case report: Favipiravir-induced bluish corneal discoloration in infant with COVID-19". Frontiers in Pediatrics 11: 1154814. doi:10.3389/fped.2023.1154814. ISSN 2296-2360. PMID 37152312.
- ↑ 11.0 11.1 "The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5'-triphosphate towards influenza A virus polymerase". PLOS ONE 8 (7): e68347. 2013. doi:10.1371/journal.pone.0068347. PMID 23874596. Bibcode: 2013PLoSO...868347J.
- ↑ "Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques". PLOS Medicine 15 (3): e1002535. March 2018. doi:10.1371/journal.pmed.1002535. PMID 29584730.
- ↑ "Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells". The Journal of Antimicrobial Chemotherapy 64 (4): 741–746. October 2009. doi:10.1093/jac/dkp274. PMID 19643775.
- ↑ "Ebola Drug From Japan May Emerge Among Key Candidates". Bloomberg.com. 7 August 2014. https://www.bloomberg.com/news/2014-08-07/ebola-drug-from-japan-may-emerge-among-key-candidates.html.
- ↑ "Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses". Antimicrobial Agents and Chemotherapy 62 (8): e00766–18. August 2018. doi:10.1128/AAC.00766-18. PMID 29891600.
- ↑ "MediVector Completes Patient Enrollment In Two Phase 3 Studies Of Favipiravir For Influenza". https://www.biospace.com/article/releases/medivector-completes-patient-enrollment-in-two-phase-3-studies-of-favipiravir-for-influenza-/.
- ↑ "Favipiravir and Zanamivir Cleared Infection with Influenza B in a Severely Immunocompromised Child". Clinical Infectious Diseases 71 (7): e191–e194. October 2020. doi:10.1093/cid/ciaa023. PMID 32124919.
- ↑ "Influenza virus polymerase inhibitors in clinical development". Current Opinion in Infectious Diseases 32 (2): 176–186. April 2019. doi:10.1097/QCO.0000000000000532. PMID 30724789.

- ↑ "Error: no
|title=specified when using {{Cite web}}" (in ja). 25 February 2014. https://diamond.jp/articles/-/49229. - ↑ "تعرف على علاج كورونا المطروح بالصيدليات المصرية وسعره ومدى نجاعته". Al Jazeera. 11 March 2021. https://www.aljazeera.net/news/healthmedicine/2021/3/11/%D8%AA%D8%B9%D8%B1%D9%81-%D8%B9%D9%84%D9%89-%D8%B9%D9%84%D8%A7%D8%AC-%D9%83%D9%88%D8%B1%D9%88%D9%86%D8%A7-%D8%A7%D9%84%D9%85%D8%B7%D8%B1%D9%88%D8%AD.
- ↑ "A kórházakat tehermentesítheti az egyforintos koronavírus-gyógyszer" (in hu). 11 March 2021. https://telex.hu/koronavirus/2021/03/11/koronavirus-elleni-gyogyszer-favipiravir-haziorvos-feliras-klinikai-teszt-hatasossag.
- ↑ "Korona virus: Koji se lekovi protiv Kovida-19 koriste u Srbiji". 22 November 2021. https://www.bbc.com/serbian/lat/srbija-58958505.
- ↑ "EVA Pharma Announces Availability of Antiviral Avipiravir® Tablets in Egyptian Pharmacies". https://web.evapharma.com/eva-pharma-announces-availability-of-antiviral-avipiravir-tablets-in-egyptian-pharmacies/.
- ↑ "'FabiFlu is the most economical COVID-19 treatment option': Glenmark's reply to Centre on alleged 'overpricing'". 21 July 2020. https://www.dnaindia.com/health/report-fabiflu-is-the-most-economical-covid-19-treatment-option-glenmark-s-reply-to-centre-on-alleged-overpricing-2833294.
- ↑ "Favipira - Tablet - 200 mg - Beacon Pharmaceuticals Ltd. - Indications, Pharmacology, Dosage, Side Effects & other Generic Info". https://medex.com.bd/brands/28003/favipira-200mg.
- ↑ "Broad-Spectrum Oral Antiviral Sales Surge for COVID-19 Treatment". https://www.precisionvaccinations.com/2021/10/29/broad-spectrum-oral-antiviral-sales-surge-covid-19-treatment.
- ↑ "Favipiravir". https://www.appilitherapeutics.com/favipiravir.
- ↑ "Russian firm gets approval for drug said to block coronavirus replication". July 8, 2020. https://in.reuters.com/article/us-health-coronavirus-russia-drug-idINKBN2492RF.
- ↑ "Russia approves R-Pharm's Coronavir for Covid-19 treatment". 9 July 2020. https://www.pharmaceutical-technology.com/news/russia-nod-coronavir-covid-19/.
- ↑ "Russia approves first COVID-19 prescription drug for sale in pharmacies" (in fr). Reuters. 2020-09-18. https://fr.reuters.com/article/us-health-coronavirus-russia-rpharm-idUSKBN26917Z.[|permanent dead link|dead link}}]
- ↑ "Japan's Drug Regulation During the COVID-19 Pandemic: Lessons From a Case Study of Favipiravir". Clinical Pharmacology and Therapeutics 111 (3): 545–547. March 2022. doi:10.1002/cpt.2251. PMID 33882157.
- ↑ "Russia approves first COVID-19 prescription drug for sale in pharmacies". Reuters. 18 September 2020. https://www.reuters.com/article/us-health-coronavirus-russia-rpharm-idUSKBN26917Z.
- ↑ "Is Favipiravir Good for COVID-19? Clinical Trial Says No, Press Release Says Yes". 25 November 2020. https://science.thewire.in/the-sciences/favipiravir-glenmark-open-label-trial-primary-endpoints-efficacy-cure-times-misleading-press-release/.
- ↑ "More patients to be given Favipiravir". 8 August 2021. https://www.bangkokpost.com/thailand/general/2161667/all-patients-to-be-given-favipiravir.
- ↑ "Scientists criticize use of unproven COVID drugs in India". Nature 587 (7833): 187–188. November 2020. doi:10.1038/d41586-020-03105-7. PMID 33169025. Bibcode: 2020Natur.587..187V.
- ↑ "Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis". Virology Journal 17 (1): 141. September 2020. doi:10.1186/s12985-020-01412-z. PMID 32972430.
- ↑ "Favipiravir to be investigated as a possible COVID-19 treatment for at-home recovery in the PRINCIPLE trial". 8 April 2021. https://www.principletrial.org/news/favipiravir-to-be-investigated-as-a-possible-covid-19-treatment-for-at-home-recovery-in-the-principle-trial. "Led by University of Oxford researchers, PRINCIPLE is one of the UK Government's national priority platform trials for COVID-19 treatments and was set-up with the intention that drugs shown to have a clinical benefit could be rapidly introduced into routine NHS care."
- ↑ "The 2014 Ebola virus disease outbreak in West Africa". The Journal of General Virology 95 (Pt 8): 1619–1624. August 2014. doi:10.1099/vir.0.067199-0. PMID 24795448.
- ↑ "Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model". Antiviral Research 105: 17–21. May 2014. doi:10.1016/j.antiviral.2014.02.014. PMID 24583123.
- ↑ "Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model". Antiviral Research 104: 153–155. April 2014. doi:10.1016/j.antiviral.2014.01.012. PMID 24462697.
- ↑ "First French Ebola patient leaves hospital". Reuters. 4 October 2016. https://www.reuters.com/article/us-health-ebola-france-idUSKCN0HT0D720141004.
- ↑ "Guinea: Clinical Trial for Potential Ebola Treatment Started in MSF Clinic in Guinea". AllAfrica – All the Time. http://allafrica.com/stories/201412260651.html.
- ↑ "Favipiravir in Patients with Ebola Virus Disease: Early Results of the JIKI trial in Guinea". CROIconference.org. 24 February 2015. http://www.croiconference.org/sessions/favipiravir-patients-ebola-virus-disease-early-results-jiki-trial-guinea.
- ↑ "Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea". PLOS Medicine 13 (3): e1001967. March 2016. doi:10.1371/journal.pmed.1001967. PMID 26930627.
- ↑ "Ebola Drug Aids Some in a Study in West Africa". The New York Times. 4 February 2015. https://www.nytimes.com/2015/02/05/science/ebola-drug-has-encouraging-early-results-and-questions-follow.html.
- ↑ "Results from encouraging Ebola trial scrutinized". Science. 26 February 2015. doi:10.1126/science.aaa7912. https://www.science.org/content/article/results-encouraging-ebola-trial-scrutinized. Retrieved 21 January 2016.
- ↑ "Nipah virus disease: A rare and intractable disease". Intractable & Rare Diseases Research 8 (1): 1–8. February 2019. doi:10.5582/irdr.2018.01130. PMID 30881850.
- ↑ "Favipiravir (T-705) protects against Nipah virus infection in the hamster model". Scientific Reports 8 (1): 7604. May 2018. doi:10.1038/s41598-018-25780-3. PMID 29765101. Bibcode: 2018NatSR...8.7604D.
- ↑ "T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections". Antiviral Research 82 (3): 95–102. June 2009. doi:10.1016/j.antiviral.2009.02.198. PMID 19428599.
- ↑ "Favipiravir (T-705), a novel viral RNA polymerase inhibitor". Antiviral Research 100 (2): 446–454. November 2013. doi:10.1016/j.antiviral.2013.09.015. PMID 24084488.
- ↑ "Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever". PLOS Neglected Tropical Diseases 8 (4): e2790. April 2014. doi:10.1371/journal.pntd.0002790. PMID 24722586.
- ↑ "Zika Virus: Where Is the Treatment?". Current Treatment Options in Infectious Diseases 8 (3): 208–211. 2016. doi:10.1007/s40506-016-0083-7. PMID 27547128.
- ↑ "Efficacy of Favipiravir (T-705) in Rabies Postexposure Prophylaxis". The Journal of Infectious Diseases 213 (8): 1253–1261. April 2016. doi:10.1093/infdis/jiv586. PMID 26655300.
- ↑ "Human Rabies - Virginia, 2017" (in en-us). MMWR. Morbidity and Mortality Weekly Report 67 (5152): 1410–1414. January 2019. doi:10.15585/mmwr.mm675152a2. PMID 30605446.
- ↑ "Favipiravir tautomerism: a theoretical insight". Theoretical Chemistry Accounts 139 (8): 145. 2020. doi:10.1007/s00214-020-02656-2. PMID 32834770.
- ↑ "Favipiravir-Tautomeric and Complexation Properties in Solution". Pharmaceuticals 16 (1): 45. December 2022. doi:10.3390/ph16010045. PMID 36678542.
External links
- "Favipiravir". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/favipiravir.
 |