Physics:Resting state fMRI
| Resting state fMRI | |
|---|---|
| Medical diagnostics | |
| File:Temporal-Non-Local-Means-Filtering-Reveals-Real-Time-Whole-Brain-Cortical-Interactions-in-Resting-pone.0158504.s002.ogv Movie of the in vivo BOLD signal from the cortical surface of a human subject from HCP, acquired using resting state fMRI, pre-processed to suppress the noise in data[1][2] and played back at a real-time rate. The BOLD signal intensities are visualized on a smoothed cortical surface. At each point on the cortex, white color represents the average BOLD signal, while blue and red colors represents lower and higher signal than average BOLD signal respectively.[2] | |
| Purpose | Evaluate regional interactions that occur in resting state(brain mapping) |
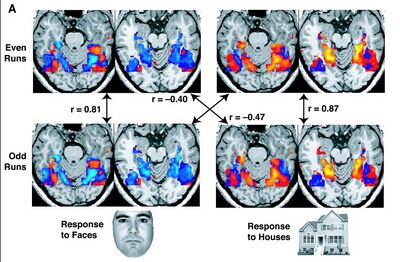
Resting state fMRI (rs-fMRI or R-fMRI) is a method of functional magnetic resonance imaging (fMRI) that is used in brain mapping to evaluate regional interactions that occur in a resting or task-negative state, when an explicit task is not being performed.[3][4] A number of resting-state brain networks have been identified, one of which is the default mode network.[5] These brain networks are observed through changes in blood flow in the brain which creates what is referred to as a blood-oxygen-level dependent (BOLD) signal that can be measured using fMRI.
Because brain activity is intrinsic, present even in the absence of an externally prompted task, any brain region will have spontaneous fluctuations in BOLD signal. The resting state approach is useful to explore the brain's functional organization and to examine if it is altered in neurological or mental disorders. Because of the resting state aspect of this imaging, data can be collected from a range of patient groups including people with intellectual disabilities, pediatric groups, and even those that are unconscious.[6][7] Resting-state functional connectivity research has revealed a number of networks which are consistently found in healthy subjects, different stages of consciousness and across species, and represent specific patterns of synchronous activity.[8][9][10]
Basics of resting state fMRI
Functional magnetic resonance imaging (functional MRI or fMRI) is a specific magnetic resonance imaging (MRI) procedure that measures brain activity by detecting associated changes in blood flow. More specifically, brain activity is measured through low frequency BOLD signal in the brain.[11]
The procedure is similar to MRI but uses the change in magnetization between oxygen-rich and oxygen-poor blood as its basic measure. This measure is frequently corrupted by noise from various sources and hence statistical procedures are used to extract the underlying signal. The resulting brain activation can be presented graphically by color-coding the strength of activation across the brain or the specific region studied. The technique can localize activity to within millimeters but, using standard techniques, no better than within a window of a few seconds.[12]
FMRI is used both in research, and to a lesser extent, in clinical settings. It can also be combined and complemented with other measures of brain physiology such as EEG, NIRS, and functional ultrasound.[13][14] Arterial spin labeling fMRI can be used as a complementary approach for assessing resting brain functions.[15] [16]
Physiological basis
The physiological blood-flow response largely decides the temporal sensitivity, how well neurons that are active can be measured in BOLD fMRI. The basic time resolution parameter is the sampling rate, or TR, which dictates how often a particular brain slice is excited and allowed to lose its magnetization. TRs could vary from the very short (500 ms) to the very long (3 seconds). For fMRI specifically, the haemodynamic response is assumed to last over 10 seconds, rising multiplicatively (that is, as a proportion of current value), peaking at 4 to 6 seconds, and then falling multiplicatively. Changes in the blood-flow system, the vascular system, integrate responses to neuronal activity over time. Because this response is a smooth continuous function, sampling with faster TRs helps only to map faster fluctuations like respiratory and heart rate signals.[17]
While fMRI strives to measure the neuronal activity in the brain, the BOLD signal can be influenced by many other physiological factors other than neuronal activity. For example, respiratory fluctuations and cardiovascular cycles affect the BOLD signal being measured in the brain and therefore are usually tried to be removed during processing of the raw fMRI data. Due to these sources of noise, there have been many experts who have approached the idea of resting state fMRI very skeptically during the early uses of fMRI. It has only been very recently that researchers have become confident that the signal being measured is not an artifact caused by other physiological function.[18]
Resting state functional connectivity between spatially distinct brain regions reflects the repeated history of co-activation patterns within these regions, thereby serving as a measure of plasticity.[19]
History
Bharat Biswal
In 1992, Bharat Biswal started his work as a graduate student at The Medical College of Wisconsin under the direction of his advisor, James S. Hyde, and discovered that the brain, even during rest, contains information about its functional organization. He had used fMRI to study how different regions of the brain communicate while the brain is at rest and not performing any active task. Though at the time, Biswal's research was mostly disregarded and attributed to another signal source, his resting neuroimaging technique has now been widely replicated and considered a valid method of mapping functional brain networks. Mapping the brain's activity while it is at rest holds many potentials for brain research and even helps doctors diagnose various diseases of the brain.[3]
Marcus Raichle
Experiments by neurologist Marcus Raichle's lab at Washington University School of Medicine and other groups showed that the brain's energy consumption is increased by less than 5% of its baseline energy consumption while performing a focused mental task. These experiments showed that the brain is constantly active with a high level of activity even when the person is not engaged in focused mental work (the resting state). His lab has been primarily focused on finding the basis of this resting activity and is credited with many groundbreaking discoveries. These include the relative independence of blood flow and oxygen consumption during changes in brain activity, which provided the physiological basis of fMRI, as well the discovery of the well known Default Mode Network.[20]
Connectivity
Functional
Functional connectivity is the connectivity between brain regions that share functional properties. More specifically, it can be defined as the temporal correlation between spatially remote neurophysiological events, expressed as deviation from statistical independence across these events in distributed neuronal groups and areas.[21] This applies to both resting state and task-state studies. While functional connectivity can refer to correlations across subjects, runs, blocks, trials, or individual time points, resting state functional connectivity focuses on connectivity assessed across individual BOLD time points during resting conditions.[22] Functional connectivity has also been evaluated using the perfusion time series sampled with arterial spin labeled perfusion fMRI.[23] Functional connectivity MRI (fcMRI), which can include resting state fMRI and task-based MRI, might someday help provide more definitive diagnoses for mental health disorders such as bipolar disorder and may also aid in understanding the development and progression of post-traumatic stress disorder as well as evaluate the effect of treatment.[24] Functional connectivity has been suggested to be an expression of the network behavior underlying high level cognitive function partially because unlike structural connectivity, functional connectivity often changes on the order of seconds as in the case of dynamic functional connectivity.[citation needed]
Networks

Default mode network
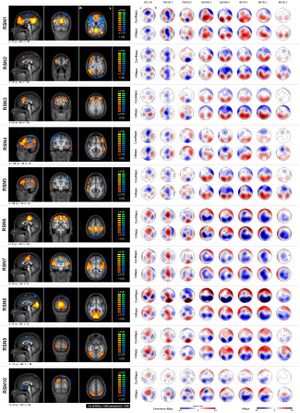
The default mode network (DMN) is a network of brain regions that are active when an individual is awake and at rest.[25] The default mode network is an interconnected and anatomically defined brain system that preferentially activates when individuals focus on internal tasks such as daydreaming, envisioning the future, retrieving memories, and gauging others' perspectives.[26] It is negatively correlated with brain systems that focus on external visual signals. It is one of the most studied networks present during resting state and is one of the most easily visualized networks.[27]
Other resting state networks
Depending on the method of resting state analysis, functional connectivity studies have reported a number of neural networks that result to be strongly functionally connected during rest. The key networks, also referred as components, which are more frequently reported include: the DMN, the sensory/motor networks, the central executive network (CEN), up to three different visual networks, a ventral and dorsal attention network, the auditory network and the limbic network.[28] As already reported, these resting-state networks consist of anatomically separated, but functionally connected regions displaying a high level of correlated BOLD signal activity. These networks are found to be quite consistent across studies, despite differences in the data acquisition and analysis techniques.[28][29] Importantly, most of these resting-state components represent known functional networks, that is, regions that are known to share and support cognitive functions.[9]
Analyzing data
Processing data
Many programs exist for the processing and analyzing of resting state fMRI data. Some of the most commonly used programs include SPM, AFNI, FSL (esp. Melodic for ICA), CONN, C-PAC, and Connectome Computation System (CCS).
Methods of analysis
There are many methods of both acquiring and processing rsfMRI data. The most popular methods of analysis focus either on independent components or on regions of correlation.[citation needed]
Independent component analysis
Independent component analysis (ICA) is a useful statistical approach in the detection of resting state networks. ICA separates a signal into non-overlapping spatial and time components. It is highly data-driven and allows for better removal of noisy components of the signal (motion, scanner drift, etc.). It also has been shown to reliably extract default mode network as well as many other networks with very high consistency.[30][31] ICA remains in the forefront of the research methods.[32]
Regional analysis
Other methods of observing networks and connectivity in the brain include the seed-based d mapping and region of interest (ROI) methods of analysis. In these cases, signal from only a certain voxel or cluster of voxels known as the seed or ROI are used to calculate correlations with other voxels of the brain. This provides a much more precise and detailed look at specific connectivity in brain areas of interest.[33][34][35] This can also be performed across the entire brain by utilizing an atlas, making it easier to define ROI's and measure connectivity. In 2021, Yeung and colleagues conducted a regional analysis utilizing a modified version of the Human Connectome Project (HCP) atlas, and found changes in the functional connectome of stroke patients during rehabilitative treatment.[36] Overall connectivity between an ROI (such as the prefrontal cortex) and all other voxels of the brain can also be averaged, providing a measure of global brain connectivity (GBC) specific to that ROI.[37] Other methods for characterizing resting-state networks include partial correlation, coherence and partial coherence, phase relationships, dynamic time warping distance, clustering, and graph theory.[38][39][40]
Reliability and reproducibility
Resting-state functional magnetic resonance imaging (rfMRI) can image low-frequency fluctuations in the spontaneous brain activities, representing a popular tool for macro-scale functional connectomics to characterize inter-individual differences in normal brain function, mind-brain associations, and the various disorders. This suggests reliability and reproducibility for commonly used rfMRI-derived measures of the human brain functional connectomics. These metrics hold great potentials of accelerating biomarker identification for various brain diseases, which call the need of addressing reliability and reproducibility at first place.[41]
Combining imaging techniques
fMRI with DWI
With fMRI providing functional and DWI structural information about the brain, these two imaging techniques are commonly used in conjunction to provide a holistic view of brain network interactions. When collected from defined ROI's, fMRI data informs researchers of how activity (blood flow) in the brain changes over time or during a task.[42] This is then bolstered through structural DWI data, which shows how individual white matter tracts connect these ROI's.[43] Investigations harnessing these techniques have progressed the field of network neuroscience, by further defining groups of regions in the brain which connect both structurally (having white matter tracts pass between them), and functionally (showing similar or opposite patterns of activity over time), into brain networks like the DMN.[44]
This combined data provides unique clinical and neuropsychiatric benefit, by enabling the investigation of how brain networks are disturbed, or white matter pathways compromised, by the presence of mental illness or structural damage.[45] Altered brain network connectivity has been shown across a swathe of disorders, such as Schizophrenia,[46][47] Depression,[48][49] Stroke,[49][50] and brain tumor,[51] underpinning their unique symptoms.
fMRI with EEG
Many imaging experts [who?] feel that in order to obtain the best combination of spatial and temporal information from brain activity, both fMRI as well as electroencephalography (EEG) should be used simultaneously. This dual technique combines the EEG's well documented ability to characterize certain brain states with high temporal resolution and to reveal pathological patterns, with fMRI's (more recently discovered and less well understood) ability to image blood dynamics through the entire brain with high spatial resolution. Up to now, EEG-fMRI has been mainly seen as an fMRI technique in which the synchronously acquired EEG is used to characterize brain activity ('brain state') across time allowing to map (through statistical parametric mapping, for example) the associated haemodynamic changes.[52]
The clinical value of these findings is the subject of ongoing investigations, but recent researches suggest an acceptable reliability for EEG-fMRI studies and better sensitivity in higher field scanner. Outside the field of epilepsy, EEG-fMRI has been used to study event-related (triggered by external stimuli) brain responses and provided important new insights into baseline brain activity during resting wakefulness and sleep.[53]
fMRI with TMS
Transcranial magnetic stimulation (TMS) uses small and relatively precise magnetic fields to stimulate regions of the cortex without dangerous invasive procedures. When these magnetic fields stimulate an area of the cortex, focal blood flow increases at the site of stimulation as well as at distant sites anatomically connected to the stimulated location. Positron emission tomography (PET) can then be used to image the brain and changes in blood flow and results show very similar regions of connectivity confirming networks found in fMRI studies and TMS can also be used to support and provide more detailed information on the connected regions.[54]
Potential pitfalls
Potential pitfalls when using rsfMRI to determine functional network integrity are contamination of the BOLD signal by sources of physiological noise such as heart rate, respiration,[55][56] and head motion.[57][58][59][60] These confounding factors can often bias results in studies where patients are compared to healthy controls in the direction of hypothesized effects, for example a lower coherence might be found in the default network in the patient group, while the patient groups also moved more during the scan. Also, it has been shown that the use of global signal regression can produce artificial correlations between a small number of signals (e.g., two or three).[61] Fortunately, the brain has many signals.[62]
Current and future applications
Research using resting state fMRI has the potential to be applied in clinical context, including use in the assessment of many different diseases and mental disorders.[63]
Disease condition and changes in resting state functional connectivity
- Alzheimer's disease: decreased connectivity[64]
- Mild cognitive impairment: abnormal connectivity[65]
- Autism: altered connectivity[66][67]
- Depression and effects of antidepressant treatment: abnormal connectivity[68][69][70][71]
- Bipolar disorder and effects of mood stabilizers: abnormal connectivity and network properties[72][73][74][75]
- Schizophrenia: disrupted networks[76]
- Attention deficit hyperactivity disorder (ADHD): altered "small networks" and thalamus changes[77]
- Aging brain: disruption of brain systems and motor network[64]
- Epilepsy: disruption and decrease/increase in connectivity[78]
- Parkinson's disease: altered connectivity[79]
- Obsessive compulsive disorder: increase/decrease in connectivity[80]
- Pain disorder: altered connectivity[81][82]
- Anorexia nervosa: connectivity alterations within corticolimbic circuitry and of insular cortex[83]
Other types of current and future clinical applications for resting state fMRI include identifying group differences in brain disease, obtaining diagnostic and prognostic information, longitudinal studies and treatment effects, clustering in heterogeneous disease states, and pre-operative mapping and targeting intervention.[84] Due to its lack of reliance on task performance and cognitive demands, resting state fMRI can be a useful tool in assessing brain alterations in disorders of impaired consciousness and cognition, as well as paediatric populations.[85]
See also
References
- ↑ "Resting-state fMRI in the Human Connectome Project". NeuroImage 80: 144–168. October 2013. doi:10.1016/j.neuroimage.2013.05.039. PMID 23702415.
- ↑ 2.0 2.1 "Temporal Non-Local Means Filtering Reveals Real-Time Whole-Brain Cortical Interactions in Resting fMRI". PLOS ONE 11 (7): e0158504. 2016-07-08. doi:10.1371/journal.pone.0158504. PMID 27391481. Bibcode: 2016PLoSO..1158504B.
- ↑ 3.0 3.1 "Resting state fMRI: a personal history". NeuroImage 62 (2): 938–944. August 2012. doi:10.1016/j.neuroimage.2012.01.090. PMID 22326802.
- ↑ "Opportunities and limitations of intrinsic functional connectivity MRI". Nature Neuroscience 16 (7): 832–837. July 2013. doi:10.1038/nn.3423. PMID 23799476.
- ↑ "Effective Connectivity within the Default Mode Network: Dynamic Causal Modeling of Resting-State fMRI Data". Frontiers in Human Neuroscience 10: 14. 2016. doi:10.3389/fnhum.2016.00014. PMID 26869900.
- ↑ "Resting state connectivity differences in eyes open versus eyes closed conditions". Human Brain Mapping 40 (8): 2488–2498. June 2019. doi:10.1002/hbm.24539. PMID 30720907.
- ↑ "Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks". The Neuroradiology Journal 30 (4): 305–317. August 2017. doi:10.1177/1971400917697342. PMID 28353416.
- ↑ "Resting State Functional Connectivity". Biological Psychiatry 69 (9): 200S. 2011. doi:10.1016/j.biopsych.2011.03.032.
- ↑ 9.0 9.1 "Resting-state brain networks: literature review and clinical applications". Neurological Sciences 32 (5): 773–785. October 2011. doi:10.1007/s10072-011-0636-y. PMID 21667095.
- ↑ "Advances and pitfalls in the analysis and interpretation of resting-state FMRI data". Frontiers in Systems Neuroscience 4: 8. 2010. doi:10.3389/fnsys.2010.00008. PMID 20407579.
- ↑ "Functional magnetic resonance imaging (FMRI) of the human brain". Journal of Neuroscience Methods 54 (2): 171–187. October 1994. doi:10.1016/0165-0270(94)90191-0. PMID 7869750.
- ↑ "Seven topics in functional magnetic resonance imaging". Journal of Integrative Neuroscience 8 (3): 371–403. September 2009. doi:10.1142/s0219635209002186. PMID 19938211.
- ↑ "Functional MRI today". International Journal of Psychophysiology 63 (2): 138–145. February 2007. doi:10.1016/j.ijpsycho.2006.03.016. PMID 16842871.
- ↑ "Synchronous multiscale neuroimaging environment for critically sampled physiological analysis of brain function: hepta-scan concept". Brain Connectivity 4 (9): 677–689. November 2014. doi:10.1089/brain.2014.0258. PMID 25131996.
- ↑ "Mapping resting-state functional connectivity using perfusion MRI". NeuroImage 40 (4): 1595–1605. May 2008. doi:10.1016/j.neuroimage.2008.01.006. PMID 18314354.
- ↑ Bertolo, Adrien (2023). "High sensitivity mapping of brain-wide functional networks in awake mice using simultaneous multi-slice fUS imaging". Imaging Neuroscience 1: 1–18. doi:10.1162/imag_a_00030.
- ↑ Functional magnetic resonance imaging (2nd ed.). Sunderland, Mass.: Sinauer Associates. 2008. ISBN 978-0-87893-286-3.
- ↑ "Consistent resting-state networks across healthy subjects". Proceedings of the National Academy of Sciences of the United States of America 103 (37): 13848–13853. September 2006. doi:10.1073/pnas.0601417103. PMID 16945915. Bibcode: 2006PNAS..10313848D.
- ↑ "Resting-state fMRI: a window into human brain plasticity". The Neuroscientist 20 (5): 522–533. October 2014. doi:10.1177/1073858414524442. PMID 24561514.
- ↑ "The human brain is intrinsically organized into dynamic, anticorrelated functional networks". Proceedings of the National Academy of Sciences of the United States of America 102 (27): 9673–9678. July 2005. doi:10.1073/pnas.0504136102. PMID 15976020. Bibcode: 2005PNAS..102.9673F.
- ↑ "Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps". NMR in Biomedicine 10 (4–5): 165–170. 1997. doi:10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. PMID 9430343.
- ↑ "Causal modelling and brain connectivity in functional magnetic resonance imaging". PLOS Biology 7 (2): e33. February 2009. doi:10.1371/journal.pbio.1000033. PMID 19226186.
- ↑ "Effects on resting cerebral blood flow and functional connectivity induced by metoclopramide: a perfusion MRI study in healthy volunteers". British Journal of Pharmacology 163 (8): 1639–1652. August 2011. doi:10.1111/j.1476-5381.2010.01161.x. PMID 21175574.
- ↑ "The future of FMRI connectivity". NeuroImage 62 (2): 1257–1266. August 2012. doi:10.1016/j.neuroimage.2012.01.022. PMID 22248579.
- ↑ "The brain's default mode network". Annual Review of Neuroscience 38 (1): 433–447. July 2015. doi:10.1146/annurev-neuro-071013-014030. PMID 25938726.
- ↑ "Functional connectivity in the resting brain: a network analysis of the default mode hypothesis". Proceedings of the National Academy of Sciences of the United States of America 100 (1): 253–258. January 2003. doi:10.1073/pnas.0135058100. PMID 12506194. Bibcode: 2003PNAS..100..253G.
- ↑ "The serendipitous discovery of the brain's default network". NeuroImage 62 (2): 1137–1145. August 2012. doi:10.1016/j.neuroimage.2011.10.035. PMID 22037421.
- ↑ 28.0 28.1 "Consistency of network modules in resting-state FMRI connectome data". PLOS ONE 7 (8): e44428. 2012. doi:10.1371/journal.pone.0044428. PMID 22952978. Bibcode: 2012PLoSO...744428M.
- ↑ "Clustering of resting state networks". PLOS ONE 7 (7): e40370. 2012. doi:10.1371/journal.pone.0040370. PMID 22792291. Bibcode: 2012PLoSO...740370L.
- ↑ "Independent component analysis of nondeterministic fMRI signal sources". NeuroImage 19 (2 Pt 1): 253–260. June 2003. doi:10.1016/S1053-8119(03)00097-1. PMID 12814576.
- ↑ "Investigations into resting-state connectivity using independent component analysis". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 360 (1457): 1001–1013. May 2005. doi:10.1098/rstb.2005.1634. PMID 16087444.
- ↑ "Ten Key Observations on the Analysis of Resting-state Functional MR Imaging Data Using Independent Component Analysis". Neuroimaging Clinics of North America 27 (4): 561–579. November 2017. doi:10.1016/j.nic.2017.06.012. PMID 28985929.
- ↑ "Connectivity-based parcellation of normal and anatomically distorted human cerebral cortex". Human Brain Mapping 43 (4): 1358–1369. November 2021. doi:10.1002/hbm.25728. PMID 34826179.
- ↑ "Mapping the functional connectivity of anterior cingulate cortex". NeuroImage 37 (2): 579–588. August 2007. doi:10.1016/j.neuroimage.2007.05.019. PMID 17604651.
- ↑ "Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization". Journal of Neurophysiology 103 (1): 297–321. January 2010. doi:10.1152/jn.00783.2009. PMID 19889849.
- ↑ "Changes in the Brain Connectome Following Repetitive Transcranial Magnetic Stimulation for Stroke Rehabilitation". Cureus 13 (10): e19105. October 2021. doi:10.7759/cureus.19105. PMID 34858752.
- ↑ "Global connectivity of prefrontal cortex predicts cognitive control and intelligence". The Journal of Neuroscience 32 (26): 8988–8999. June 2012. doi:10.1523/JNEUROSCI.0536-12.2012. PMID 22745498.
- ↑ "Time-frequency dynamics of resting-state brain connectivity measured with fMRI". NeuroImage 50 (1): 81–98. March 2010. doi:10.1016/j.neuroimage.2009.12.011. PMID 20006716.
- ↑ "Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies". NeuroImage 61 (3): 613–621. July 2012. doi:10.1016/j.neuroimage.2012.03.078. PMID 22498656.
- ↑ "Resting State fMRI Functional Connectivity Analysis Using Dynamic Time Warping". Frontiers in Neuroscience 11: 75. 2017-01-01. doi:10.3389/fnins.2017.00075. PMID 28261052.
- ↑ "Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective". Neuroscience and Biobehavioral Reviews 45: 100–118. September 2014. doi:10.1016/j.neubiorev.2014.05.009. PMID 24875392.
- ↑ "The human brain is intrinsically organized into dynamic, anticorrelated functional networks". Proceedings of the National Academy of Sciences of the United States of America 102 (27): 9673–9678. July 2005. doi:10.1073/pnas.0504136102. PMID 15976020. Bibcode: 2005PNAS..102.9673F.
- ↑ "Diffusion weighted imaging: Technique and applications". World Journal of Radiology 8 (9): 785–798. September 2016. doi:10.4329/wjr.v8.i9.785. PMID 27721941.
- ↑ "A Connectomic Atlas of the Human Cerebrum-Chapter 1: Introduction, Methods, and Significance". Operative Neurosurgery 15 (suppl_1): S1–S9. December 2018. doi:10.1093/ons/opy253. PMID 30260422.
- ↑ "Reducing the Cognitive Footprint of Brain Tumor Surgery". Frontiers in Neurology 12: 711646. 2021. doi:10.3389/fneur.2021.711646. PMID 34484105.
- ↑ "Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon?". Schizophrenia Bulletin 34 (1): 72–92. January 2008. doi:10.1093/schbul/sbm034. PMID 17485733.
- ↑ "Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach". Biological Psychiatry. Schizophrenia: N-methyl-D-aspartate Receptor Dysfunction and Cortical Connectivity 68 (1): 61–69. July 2010. doi:10.1016/j.biopsych.2010.03.035. PMID 20497901.
- ↑ "Disrupted structural connectivity network in treatment-naive depression". Progress in Neuro-Psychopharmacology & Biological Psychiatry 56: 18–26. January 2015. doi:10.1016/j.pnpbp.2014.07.007. PMID 25092218.
- ↑ 49.0 49.1 "Abnormal Functional and Structural Connectivity of Amygdala-Prefrontal Circuit in First-Episode Adolescent Depression: A Combined fMRI and DTI Study". Frontiers in Psychiatry 10: 983. 2020. doi:10.3389/fpsyt.2019.00983. PMID 32116814.
- ↑ "Longitudinal changes of resting-state functional connectivity during motor recovery after stroke". Stroke 42 (5): 1357–1362. May 2011. doi:10.1161/STROKEAHA.110.596155. PMID 21441147.
- ↑ "Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices". Clinical Neurophysiology 117 (9): 2039–2049. September 2006. doi:10.1016/j.clinph.2006.05.018. PMID 16859985.
- ↑ "Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat". Journal of Magnetic Resonance Imaging 30 (2): 384–393. August 2009. doi:10.1002/jmri.21848. PMID 19629982.
- ↑ "Evaluation of data-driven network analysis approaches for functional connectivity MRI". Brain Structure & Function 215 (2): 129–140. August 2010. doi:10.1007/s00429-010-0276-7. PMID 20853181.
- ↑ "Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS)". NeuroImage 62 (4): 2232–2243. October 2012. doi:10.1016/j.neuroimage.2012.03.035. PMID 22465297.
- ↑ "Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI". NeuroImage 31 (4): 1536–1548. July 2006. doi:10.1016/j.neuroimage.2006.02.048. PMID 16632379.
- ↑ "Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI". NeuroImage 47 (4): 1381–93. October 2009. doi:10.1016/j.neuroimage.2009.04.048. PMID 19393322.
- ↑ "A dual echo approach to motion correction for functional connectivity studies". NeuroImage 63 (3): 1487–1497. November 2012. doi:10.1016/j.neuroimage.2012.07.042. PMID 22846657.
- ↑ "The influence of head motion on intrinsic functional connectivity MRI". NeuroImage 59 (1): 431–438. January 2012. doi:10.1016/j.neuroimage.2011.07.044. PMID 21810475.
- ↑ "Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion". NeuroImage 59 (3): 2142–2154. February 2012. doi:10.1016/j.neuroimage.2011.10.018. PMID 22019881.
- ↑ "Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth". NeuroImage 60 (1): 623–632. March 2012. doi:10.1016/j.neuroimage.2011.12.063. PMID 22233733.
- ↑ "Trouble at rest: how correlation patterns and group differences become distorted after global signal regression". Brain Connectivity 2 (1): 25–32. 2012. doi:10.1089/brain.2012.0080. PMID 22432927.
- ↑ "Estimation of the intrinsic dimensionality of fMRI data". NeuroImage 29 (1): 145–154. January 2006. doi:10.1016/j.neuroimage.2005.07.054. PMID 16202626.
- ↑ "Functional brain networks in movement disorders: recent advances". Current Opinion in Neurology 25 (4): 392–401. August 2012. doi:10.1097/wco.0b013e328355aa94. PMID 22710361.
- ↑ 64.0 64.1 "Alterations of directional connectivity among resting-state networks in Alzheimer disease". AJNR. American Journal of Neuroradiology 34 (2): 340–5. February 2013. doi:10.3174/ajnr.A3197. PMID 22790250.
- ↑ "Three subsystems of the inferior parietal cortex are differently affected in mild cognitive impairment". Journal of Alzheimer's Disease 30 (3): 475–487. 2012. doi:10.3233/JAD-2012-111721. PMID 22451310.
- ↑ "Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders". Cerebral Cortex 21 (10): 2233–2243. October 2011. doi:10.1093/cercor/bhq296. PMID 21378114.
- ↑ "Identifying differences in brain activities and an accurate detection of autism spectrum disorder using resting state functional-magnetic resonance imaging : A spatial filtering approach". Medical Image Analysis 35: 375–389. January 2017. doi:10.1016/j.media.2016.08.003. PMID 27585835.
- ↑ "Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study". Biological Psychiatry 57 (10): 1079–1088. May 2005. doi:10.1016/j.biopsych.2005.02.021. PMID 15866546.
- ↑ "Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus". Biological Psychiatry 62 (5): 429–437. September 2007. doi:10.1016/j.biopsych.2006.09.020. PMID 17210143.
- ↑ "Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study" (in En). Neuropsychopharmacology 30 (7): 1334–1344. July 2005. doi:10.1038/sj.npp.1300725. PMID 15856081.
- ↑ "Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study". The Journal of Neuropsychiatry and Clinical Neurosciences 19 (3): 274–282. 2007-07-01. doi:10.1176/jnp.2007.19.3.274. PMID 17827412.
- ↑ "Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression". Psychiatry Research 171 (3): 189–198. March 2009. doi:10.1016/j.pscychresns.2008.03.012. PMID 19230623.
- ↑ "Resting State Brain Network Disturbances Related to Hypomania and Depression in Medication-Free Bipolar Disorder" (in En). Neuropsychopharmacology 41 (13): 3016–3024. December 2016. doi:10.1038/npp.2016.112. PMID 27356764.
- ↑ "Differential Resting-State Functional Connectivity of Striatal Subregions in Bipolar Depression and Hypomania". Brain Connectivity 6 (3): 255–265. April 2016. doi:10.1089/brain.2015.0396. PMID 26824737.
- ↑ "Lithium monotherapy associated clinical improvement effects on amygdala-ventromedial prefrontal cortex resting state connectivity in bipolar disorder". Journal of Affective Disorders 225: 4–12. January 2018. doi:10.1016/j.jad.2017.06.047. PMID 28772145.
- ↑ "Whole brain resting state functional connectivity abnormalities in schizophrenia". Schizophrenia Research 139 (1–3): 7–12. August 2012. doi:10.1016/j.schres.2012.04.021. PMID 22633528.
- ↑ "Network homogeneity reveals decreased integrity of default-mode network in ADHD". Journal of Neuroscience Methods 169 (1): 249–254. March 2008. doi:10.1016/j.jneumeth.2007.11.031. PMID 18190970.
- ↑ "Resting-state fMRI studies in epilepsy". Neuroscience Bulletin 28 (4): 449–455. August 2012. doi:10.1007/s12264-012-1255-1. PMID 22833042.
- ↑ "Resting-state brain connectivity in patients with Parkinson's disease and freezing of gait". Parkinsonism & Related Disorders 18 (6): 781–787. July 2012. doi:10.1016/j.parkreldis.2012.03.018. PMID 22510204.
- ↑ "Altered resting state functional connectivity patterns of the anterior prefrontal cortex in obsessive-compulsive disorder". NeuroReport 23 (11): 681–686. August 2012. doi:10.1097/wnr.0b013e328355a5fe. PMID 22692554.
- ↑ "Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder". BMC Psychiatry 13: 84. March 2013. doi:10.1186/1471-244x-13-84. PMID 23497482.
- ↑ "Functional network connectivity of pain-related resting state networks in somatoform pain disorder: an exploratory fMRI study". Journal of Psychiatry & Neuroscience 38 (1): 57–65. January 2013. doi:10.1503/jpn.110187. PMID 22894821.
- ↑ "A systematic review of resting-state functional-MRI studies in anorexia nervosa: Evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration". Neuroscience and Biobehavioral Reviews 71: 578–589. December 2016. doi:10.1016/j.neubiorev.2016.09.032. PMID 27725172.
- ↑ "Clinical applications of resting state functional connectivity". Frontiers in Systems Neuroscience 4: 19. 2010. doi:10.3389/fnsys.2010.00019. PMID 20592951.
- ↑ Lee, MH; Smyser, CD; Shimony, JS (October 2013). "Resting-state fMRI: a review of methods and clinical applications.". AJNR. American Journal of Neuroradiology 34 (10): 1866–72. doi:10.3174/ajnr.A3263. PMID 22936095.
 |