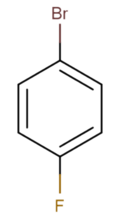
Chemistry:4-Bromofluorobenzene
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Bromo-4-fluorobenzene | |||
| Other names
p-Bromofluorobenzene
p-Fluorophenyl bromide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | PBFB | ||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C6H4BrF | |||
| Molar mass | 175.000 g·mol−1 | ||
| Appearance | Clear, slightly yellow to clear liquid | ||
| Melting point | −16 °C (3 °F; 257 K) | ||
| Boiling point | 150 °C (302 °F; 423 K) | ||
| Insoluble | |||
| Structure | |||
| Planar | |||
| Hazards | |||
| R-phrases (outdated) | R36, R37, R38 | ||
| S-phrases (outdated) | S26, S36, S37, S39 | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related halobenzenes
|
1,4-Dichlorobenzene 1,4-Dibromobenzene 1,4-Diiodobenzene | ||
Related compounds
|
Benzene 1,4-Difluorobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
4-Bromofluorobenzene is a halogenated aromatic organic compound with the formula C6H4BrF. It is a derivative of benzene, with a bromine atom bonded para to a fluorine atom. It has uses as a precursor to some pharmaceuticals, as an agrochemical intermediate, and in organic synthesis.[1]
Uses
The electronegativity (withdrawal of electron density) of the halogens present (bromine and fluorine) on 4-bromofluorobenzene cause deactivation of the benzene ring and direct reactivity to ortho and para positions on the ring.[2] This redirection of reactivity makes it useful as a precursor other compounds that serve a wide variety of needs. The bromine on 4-bromofluorobenzene allows it to become a Grignard reagent when reacted with magnesium metal.[3] Preparation of a Grignard reagent using it makes it useful as an intermediate in the synthesis of atypical antipsychotic agents (especially those with a 4-fluorophenyl moiety),[1][4] and in pesticides such as Flusilazole.[5] This compound is also used as a tuning solution, internal standard, and/or surrogate (a compound that is similar in chemical composition to the analyte(s) of interest and is spiked into environmental and batch quality control samples prior to sample preparation and analysis) in several EPA gas chromatography methods, such as those for drinking water, wastewater pollutants, and organic pollutant monitoring in groundwater, wastewater, and solid waste.[1]
Production
4-Bromofluorobenzene is regarded by the Toxic Substances Control Act as a high production volume chemical, that is, a chemical that 1 million pounds (about 500 tonnes) per year is either produced in or imported to the United States. As of 2002, companies producing or importing this compound reported 10 million (4,536,000 kilograms) used. This is up from 500,000 pounds (226,800 kilograms) used in 1986, as reported in the Inventory Update Rule of the Toxic Substances Control Act.[1]
Reactivity and synthesis
Halogenated aromatic compounds are relatively unreactive, as reactivity is generally negatively related to degree of substitution of halogen atoms for hydrogen atoms.[1] 4-Bromofluorobenzene is most commonly synthesized via electrophilic halogenation, a type of electrophilic aromatic substitution with fluorobenzene, bromine, and iron(III) bromide as the reagents.[1] The fluorine on fluorobenzene is a weak para and ortho director,[6] as it draws electron density from the meta ring positions.[7] Below is the reaction mechanism:
Safety
This compound is a flammable liquid and vapor. It causes eye irritation, and may be harmful if swallowed, inhaled, or absorbed through the skin. It can cause depression of the central nervous system. It has a flash point of 53 °C.[8]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "4-Bromofluorobenzene [CAS No. 460-00-4 Review of Toxicological Literature"]. National Toxicology Program. http://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/4-bfb_508.pdf. Retrieved 2 January 2015.
- ↑ Aromatic Reactivity
- ↑ Patent US4956507 - Grignard reagents from p-halofluorobenzene and magnesium coupling to form p ... - Google Patents
- ↑ Patent US4605655 - Neuroleptic agents; no movement disorder side effects - Google Patents
- ↑ "4-Bromofluorobenzene". Shandong A&Fine Agrochemicals CO.,Ltd. http://www.afineagro.com/cgi/search-en.cgi?f=product_en1+product_en_1_+company_en_1_+contact_en&id=576084&t=product_en_1_. Retrieved 2 January 2015.
- ↑ Ch12 : Substituent Effects
- ↑ Illustrated Glossary of Organic Chemistry - Electron withdrawing group (EWG)
- ↑ "Material Safety Data Sheet". Acros Organics. https://www.fishersci.ca/viewmsds.do?catNo=AC107011000. Retrieved 2 January 2015.