Chemistry:L-Arginine ethyl ester
| |
| Names | |
|---|---|
| IUPAC name
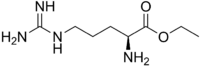
(S)-Ethyl 2-amino-5-(diaminomethylideneamino)pentanoate
| |
| Other names
Arginine ethyl ester; Ethyl l-arginate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H18N4O2 | |
| Molar mass | 202.258 g·mol−1 |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
l-Arginine ethyl ester or ethyl arginate is an alternative supplement form of the conditionally-essential amino acid arginine bound to an ethyl ester. Esters are organic compounds formed by esterification – the reaction of carboxylic acid and alcohols.[1]
Physical and chemical properties
Arginine ethyl ester falls under the category of protected aminoacids. These are modified aminoacids molecules which show a different pharmacokinetics and pharmacodynamics compared to the base form. In fact the ethyl ester in Arginine ethyl ester is bound to the hydrogen side of the molecule, protecting it from hydrolization by the enzyme arginase, which in fact attaches to arginine on this side, until the ester is cleaved by esterases enzymes. The ester also confers lipophylicity to this form of arginine, base arginine is hydrophilic, allowing it to passively penetrate cells membranes, i.e. it doesn't need the specific transporter to cross lipid membranes, in so allowing maximum tissue distribution. Many pharmaceutical compounds are attached to an ethyl ester for the very same purpose of enhancing tissues distribution. Arginine ethyl ester acts as a prodrug, the clivage of the ester by esterases yield arginine and ethanol.
Uses
l-Arginine ethyl ester is biochemical reagent which can be used as a pharmaceutical intermediate.[2]
References
- ↑ "Benzolyl-l-arginine ethyl ester" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/54188221.
- ↑ "Antiviral and virucidal activities of nα-cocoyl-L-arginine ethyl ester". Advances in Virology 2011: 572868. 2011. doi:10.1155/2011/572868. PMID 22312346.
 |

