Chemistry:Tavilermide
 | |
| Clinical data | |
|---|---|
| Routes of administration | Eye drop |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
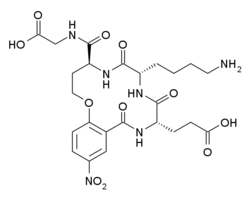
| Formula | C24H32N6O11 |
| Molar mass | 580.551 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tavilermide (INN) (developmental code name MIM-D3) is a selective, cyclic tripeptide partial agonist of TrkA. (this class of drugs is sometimes referred to as nerve growth factor (NGF) mimetics)[1][2][3] Tavilermide was first synthesized by Burgess and co-workers at Texas A&M University with the intention of producing TrkA agonists.[4][5] It is under development by Mimetogen Pharmaceuticals as an ophthalmic (eye drop) solution for the treatment of dry eyes, and is in phase III clinical trials for this indication. Tavilermide is currently being evaluated in two multi-center phase III clinical studies in the United States for the treatment of dry eye disease.[1] Tavilermide is also in phase I clinical trials for the treatment of glaucoma; studies are ongoing.[6][7]
See also
References
- ↑ "Safety and efficacy of MIM-D3 ophthalmic solutions in a randomized, placebo-controlled Phase 2 clinical trial in patients with dry eye". Clinical Ophthalmology 7: 1275–1285. 2013. doi:10.2147/OPTH.S44688. PMID 23836957.
- ↑ "An NGF mimetic, MIM-D3, stimulates conjunctival cell glycoconjugate secretion and demonstrates therapeutic efficacy in a rat model of dry eye". Experimental Eye Research 93 (4): 503–512. October 2011. doi:10.1016/j.exer.2011.06.014. PMID 21726552.
- ↑ "The Future of Dry Eye Treatment: A Glance into the Therapeutic Pipeline". Ophthalmology and Therapy 4 (2): 69–78. December 2015. doi:10.1007/s40123-015-0038-y. PMID 26289997.
- ↑ "SNAr Cyclizations To Form Cyclic Peptidomimetics of β-Turns". Journal of the American Chemical Society 120 (41): 10768–10769. 1998. doi:10.1021/ja981589t. https://pubs.acs.org/doi/10.1021/ja981589t.
- ↑ "Solid-phase SNAr macrocyclizations to give turn-extended-turn peptidomimetics". Chemistry: A European Journal 5 (11): 3261–3272. 1999. doi:10.1002/(SICI)1521-3765(19991105)5:11<3261::AID-CHEM3261>3.0.CO;2-H. https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/%28SICI%291521-3765%2819991105%295%3A11%3C3261%3A%3AAID-CHEM3261%3E3.0.CO%3B2-H.
- ↑ "Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement". Ophthalmology 119 (5): 979–986. May 2012. doi:10.1016/j.ophtha.2011.11.003. PMID 22349567.
- ↑ "Tavilermide". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800033231.
External links
 |

