Chemistry:Neostigmine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Bloxiverz, Prostigmin, Vagostigmin, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intramuscular, intravenous, subcutaneous, by mouth |
| Drug class | Cholinesterase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unclear, probably less than 5% |
| Metabolism | Slow hydrolysis by acetylcholinesterase and also by plasma esterases |
| Onset of action | Within 10-20 min (injection),[3] with 4 hrs (by mouth) [citation needed] |
| Elimination half-life | 50–90 minutes |
| Duration of action | up to 4 hrs[3] |
| Excretion | Unchanged drug (up to 70%) and alcoholic metabolite (30%) are excreted in the urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
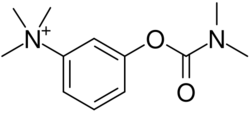
| Formula | C12H19N2O2+ |
| Molar mass | 223.296 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Neostigmine, sold under the brand name Bloxiverz, among others, is a medication used to treat myasthenia gravis, Ogilvie syndrome, and urinary retention without the presence of a blockage.[3][4] It is also used in anaesthesia to end the effects of non-depolarising neuromuscular blocking medication.[3] It is given by injection either into a vein, muscle, or under the skin.[3] After injection effects are generally greatest within 30 minutes and last up to 4 hours.[3][5]
Common side effects include nausea, increased saliva, crampy abdominal pain, and slow heart rate.[3] More severe side effects include low blood pressure, weakness, and allergic reactions.[3] It is unclear if use in pregnancy is safe for the baby.[3] Neostigmine is in the cholinergic family of medications.[3] It works by blocking the action of acetylcholinesterase and therefore increases the levels of acetylcholine.[3]
Neostigmine was patented in 1931.[6] It is on the World Health Organization's List of Essential Medicines.[7] The term is from Greek neos, meaning "new", and "-stigmine", in reference to its parent molecule, physostigmine, on which it is based.[8] It is available as a generic medication.[9]
Medical uses
It is used to improve muscle tone in people with myasthenia gravis, and also to reverse the effects of non-depolarizing muscle relaxants such as rocuronium and vecuronium at the end of an operation.[10][11]
Another indication for use is the conservative management of acute colonic pseudo-obstruction, or Ogilvie's syndrome, in which patients get massive colonic dilatation in the absence of a true mechanical obstruction.[12]
Neostigmine is often prescribed for underactive urinary bladder.[13]
Hospitals sometimes administer a solution containing neostigmine intravenously to delay the effects of envenomation through snakebite.[14] Some promising research results have also been reported for administering the drug nasally in order to buy time if anti-venom is not immediately available.[15]
Side effects
Neostigmine has a wide variety of side-effects due to its action that increases acetylcholine (ACh) binding muscarinic receptors on exocrine glandular cells throughout the body, cardiac muscle cells, and smooth muscle cells. These effects include: salivation, lacrimation, diarrhea, bradycardia, and bronchoconstriction.[16] Gastrointestinal symptoms occur earliest.[17]: 109
For this reason, it is usually given along with an anti-cholinergic drug such as atropine or glycopyrrolate which act only on muscarinic receptors while permitting neostigmine action at nicotinic receptors.[18]
Neostigmine can also induce generic ocular side effects including: headache, brow pain, blurred vision, phacodonesis, pericorneal injection, congestive iritis, various allergic reactions, and rarely, retinal detachment.[17]: 114
Pharmacology
Acetylcholine is metabolized by the enzyme acetylcholinesterase that cleaves acetylcholine in the neuromuscular junction into acetate and choline. Neostigmine is an inhibitor of acetylcholinesterase. Neostigmine binds to the anionic and ester site of acetylcholinesterase, which blocks the enzyme from breaking down the acetylcholine molecules before they reach the postsynaptic membrane receptors. Its action leads to the accumulation of acetylcholine in the neuromuscular junction that compete with the non-depolarizing blocker agent bound to the acetylcholine receptors. By interfering with the breakdown of acetylcholine, neostigmine indirectly stimulates both nicotinic and muscarinic receptors.[10]
Unlike physostigmine, neostigmine has a quaternary nitrogen; hence, it is more polar. It does not cross the blood–brain barrier and enter the CNS.[19] However, it does cross the placenta.
Neostigmine is administered intravenously. The drug should be administered when a peripheral nerve stimulator shows a second twitch is present or when the first twitch response is considerably above 10% of baseline. Peak effect is at 7 to 10 minutes.[10] Neostigmine has moderate duration of action – usually two to four hours.[20] It is metabolized by enzymes in the liver and excreted in the urine.[10]
Chemistry
Neostigmine, which can be viewed as a simplified analog of physostigmine, is made by reacting 3-dimethylaminophenol with N-dimethylcarbamoyl chloride, which forms the dimethylcarbamate, and its subsequent alkylation using dimethyl sulfate forming the desired compound.
Spectral data
Neostigmine shows notable UV/VIS absorption at 261 nm, 267 nm, and 225 nm.[21]
Neostigmine's 1H NMR Spectroscopy reveals shifts at: 7.8, 7.7, 7.4, 7.4, 3.8, and 3.1 parts per million. The higher shifts are due to the aromatic hydrogens. The lower shifts at 3.8 ppm and 3.1 ppm are due to the electronic withdrawing nature of the tertiary and quaternary nitrogen, respectively.[22]
History
Neostigmine was first synthesized by Aeschlimann and Reinert in 1931[23] and was patented by Aeschlimann in 1933.[24]
Neostigmine is made by first reacting 3-dimethylaminophenol with N-dimethylcarbamoyl chloride, which forms a dimethylcarbamate. Next, that product is alkylated using dimethyl sulfate, which forms neostigmine.[17]: 103
References
- ↑ "Neostigmine Use During Pregnancy". 3 January 2020. https://www.drugs.com/pregnancy/neostigmine.html.
- ↑ "Bloxiverz- neostigmine methylsulfate injection". 3 March 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1fb5c812-1e63-4bd9-a68e-7f9dc5e7a33a.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 "Neostigmine Bromide". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/neostigmine-bromide.html.
- ↑ WHO Model Formulary 2008. World Health Organization. 2009. p. 428. ISBN 9789241547659.
- ↑ "Neostigmine Methylsulfate Monograph for Professionals". The American Society of Health-System Pharmacists. 19 September 2019. https://www.drugs.com/monograph/neostigmine-methylsulfate.html.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 540. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA540.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "neostigmine: definition of neostigmine in Oxford dictionary (American English) (US)". http://www.oxforddictionaries.com/us/definition/american_english/neostigmine.
- ↑ "Competitive Generic Therapy Approvals". 29 June 2023. https://www.fda.gov/drugs/generic-drugs/competitive-generic-therapy-approvals.
- ↑ 10.0 10.1 10.2 10.3 "Neostigmine". StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2022. https://www.ncbi.nlm.nih.gov/books/NBK470596/.
- ↑ "Neostigmine Injection: Indications, Side Effects, Warnings". Drugs.com. https://www.drugs.com/cdi/neostigmine-injection.html.
- ↑ "Acute intestinal pseudo-obstruction (Ogilvie's syndrome)". Clinics in Colon and Rectal Surgery 18 (2): 96–101. May 2005. doi:10.1055/s-2005-870890. PMID 20011348.
- ↑ "The effectiveness of parasympathomimetics for treating underactive bladder: A systematic review and meta-analysis". Neurourology and Urodynamics 41 (1): 127–139. January 2022. doi:10.1002/nau.24839. PMID 34816481. https://pure.bond.edu.au/ws/files/118955227/AM_The_effectiveness_of_parasympathomimetics_for_treating.pdf.
- ↑ "Potential Treatment For Snakebites Leads To A Paralyzing Test". NPR. 31 July 2013. https://www.npr.org/blogs/health/2013/07/30/207050435/potential-treatment-for-snakebites-leads-to-a-paralyzing-test.
- ↑ "Developing Small Molecule Therapeutics for the Initial and Adjunctive Treatment of Snakebite". Journal of Tropical Medicine 2018: 4320175. 2018. doi:10.1155/2018/4320175. PMID 30154870.
- ↑ "Muscarinic Antagonists". StatPearls [Internet].. Treasure Island (FL): StatPearls Publishing. 2022. https://www.ncbi.nlm.nih.gov/books/NBK557541/.
- ↑ 17.0 17.1 17.2 Goodman & Gilman's The Pharmacological Basis of Therapeutics (6th ed.). New York: Macmillan Publishing Co., Inc.. 1980.
- ↑ "Glycopyrrolate: It's time to review". Journal of Clinical Anesthesia 36: 51–53. February 2017. doi:10.1016/j.jclinane.2016.09.013. PMID 28183573.
- ↑ "Addition of Neostigmine and Atropine to Conventional Management of Postdural Puncture Headache: A Randomized Controlled Trial" (in en-US). Anesthesia and Analgesia 127 (6): 1434–1439. December 2018. doi:10.1213/ANE.0000000000003734. PMID 30169405.
- ↑ Pharmacology (3rd ed.). Lippincott's Illustrated Reviews. 2008. p. 51.
- ↑ "[The structure of degradation products of neostigmine bromide]" (in de). Die Pharmazie 40 (5): 325–328. May 1985. PMID 4034636.
- ↑ "Application of the WATR technique for water suppression in 1H NMR spectroscopy in determination of the kinetics of hydrolysis of neostigmine bromide in aqueous solution". The Journal of Pharmacy and Pharmacology 45 (6): 559–562. June 1993. doi:10.1111/j.2042-7158.1993.tb05598.x. PMID 8103105.
- ↑ Reviews of Environmental Contamination and Toxicology. Springer Science+Business Media. 2007. p. 57. ISBN 978-0-387-73162-9. https://books.google.com/books?id=PCIbhO_xlcEC&pg=PA57.
- ↑ Aeschliman JA, US patent 1905990, issued 1933
External links
- "Neostigmine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/59-99-4.
- "Neostigmine methylsulfate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/neostigmine%20methylsulfate.
- "Neostigmine bromide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/neostigmine%20bromide.
 |
