Chemistry:Brimonidine
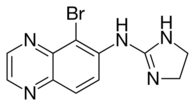 | |
| Clinical data | |
|---|---|
| Pronunciation | /brɪˈmoʊnɪdiːn/ bri-MOH-nid-een |
| Trade names | Alphagan, Mirvaso, Lumify, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601232 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Primarily liver |
| Elimination half-life | 3 hours (ocular), 12 hours (topical) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H10BrN5 |
| Molar mass | 292.140 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 252 °C (486 °F) |
| |
| |
| (verify) | |
Brimonidine is an α2 agonist medication used to treat open-angle glaucoma, ocular hypertension, and rosacea.[1][2] In rosacea it improves the redness.[2] It is used as eye drops or applied to the skin.[1][2]
Common side effects when used in the eyes include itchiness, redness, and a dry mouth.[1] Common side effects when used on the skin include redness, burning, and headaches.[2] More significant side effects may include allergic reactions and low blood pressure.[2][1] Use in pregnancy appears to be safe.[2][1] When applied to the eye it works by decreasing the amount of aqueous humor made while increasing the amount that drains from the eye.[1] When applied to the skin it works by causing blood vessels to contract.[2]
Brimonidine was patented in 1972 and came into medical use in 1996.[3] It is available as a generic medication.[4][5] In 2020, it was the 175th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[6][7]
Medical uses
Brimonidine is indicated for the lowering of intraocular pressure in patients with open-angle glaucoma or ocular hypertension. It is also the active ingredient of brimonidine/timolol along with timolol maleate.
A 2017 Cochrane review found insufficient evidence to determine if brimonidine slows optic nerve damage.[8]
In 2013, the FDA approved topical application of brimonidine 0.33% gel for persistent facial redness of rosacea.
Mechanism of action
Brimonidine is an α2 adrenergic agonist.[1]
α2 agonists, through the activation of a G protein-coupled receptor, inhibit the activity of adenylate cyclase. This reduces cAMP and hence aqueous humour production by the ciliary body.
Peripheral α2 agonist activity results in vasoconstriction of blood vessels (as opposed to central α2 agonist activity that decreases sympathetic tone, as can be seen by the medication clonidine). This vasoconstriction may explain the acute reduction in aqueous humor flow. The increased uveoscleral outflow from prolonged use may be explained by increased prostaglandin release due to α adrenergic stimulation. This may lead to relaxed ciliary muscle and increased uveoscleral outflow.[9]
Society and culture
Names
It is sold under the brand names Alphagan, Alphagan-P, Mirvaso, Lumify, Brymont, and others.
Over the counter
In July 2018, Bausch and Lomb began to market over the counter (OTC) eye drops, using brimonidine's tartrate formulation in a concentration of 0.025%, as an ophthalmic vasoconstrictor under the brand name Lumify. Intended to relieve redness in the sclerae of the eyes for periods of up to eight hours at a time through its vasoconstrictive effects, Lumify was marketed as an alternative to Visine, the brand of tetrahydrozoline hydrochloride solution most commonly used for that purpose.[citation needed]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Brimonidine Tartrate eent Monograph for Professionals" (in en). American Society of Health-System Pharmacists. https://www.drugs.com/monograph/brimonidine-tartrate-eent.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Brimonidine Tartrate topical Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/brimonidine-tartrate-topical.html.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 550. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA550.
- ↑ "Competitive Generic Therapy Approvals". 29 June 2023. https://www.fda.gov/drugs/generic-drugs/competitive-generic-therapy-approvals.
- ↑ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 1153. ISBN 9780857113382.
- ↑ "The Top 300 of 2019". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Brimonidine - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Brimonidine.
- ↑ "Neuroprotection for treatment of glaucoma in adults". The Cochrane Database of Systematic Reviews 1 (1): CD006539. January 2017. doi:10.1002/14651858.CD006539.pub4. PMID 28122126.
- ↑ "Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients". American Journal of Ophthalmology 128 (1): 8–14. July 1999. doi:10.1016/s0002-9394(99)00076-8. PMID 10482088.
Further reading
- "Brimonidine tartrate for the treatment of glaucoma". Expert Opin Pharmacother 20 (1): 115–122. January 2019. doi:10.1080/14656566.2018.1544241. PMID 30407890.
External links
- "Brimonidine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/59803-98-4.
- "Brimonidine tartrate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/brimonidine%20tartrate.
 |

