Biology:Beta-propeller
From HandWiki
| WD domain, G-beta repeat | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | WD40 | ||||||||
| Pfam | PF00400 | ||||||||
| Pfam clan | CL0186 | ||||||||
| InterPro | IPR001680 | ||||||||
| PROSITE | PDOC00574 | ||||||||
| SCOP2 | 1gp2 / SCOPe / SUPFAM | ||||||||
| CDD | cd00200 | ||||||||
| |||||||||
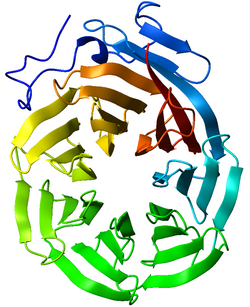
In structural biology, a beta-propeller is a type of all-β protein architecture characterized by 4 to 8 blade-shaped beta sheets arranged toroidally around a central axis. Each sheet typically has four antiparallel β-strands twisted so that the first and fourth strands are almost perpendicular to each other. The enzyme's active site is often found in the cleft formed in the center of the propeller by loops connecting the successive four-sheet motifs. Murzin proposed a geometric model to describe the structural principles of the beta propeller.[2] According to this model the seven bladed propeller was the most favoured arrangement in geometric terms.
Examples
- The influenza virus protein viral neuraminidase is a six-bladed beta-propeller protein whose active form is a tetramer. It is one of two proteins present in the viral envelope and catalyzes the cleavage of sialic acid moieties from cell-membrane proteins to aid in the targeting of newly produced virions to previously uninfected cells.
- The plant UV-B sensing protein UVR8 is a seven-bladed propeller which is dimeric until absorption of UV-B light, which causes dissociation.
- WD40 repeats, also known as beta-transducin repeats, are short fragments found primarily in eukaryotes.[3][4] They are often assembled in 4 to 16 repeated units to form a structural domain critical for protein–protein interactions.
- A beta-propeller is a critical component of LDLR (low density lipoprotein receptor) and aids in a pH based conformational change. At neutral pH the LDLR is in an extended linear conformation and can bind ligands (PCSK9). At acidic pH the linear conformation changes to a hairpin structure such that ligand binding sites bind to the beta-propeller, preventing ligand binding.[5][6]
- Beta-propeller phytases consist of a six-bladed β-propeller structure.[7]
References
- ↑ Sprague, E. R.; Redd, M. J.; Johnson, A. D.; Wolberger, C. (2000). "Structure of the C-terminal domain of Tup1, a corepressor of transcription in yeast". The EMBO Journal 19 (12): 3016–3027. doi:10.1093/emboj/19.12.3016. PMID 10856245.
- ↑ Murzin AG (October 1992). "Structural principles for the propeller assembly of beta-sheets: the preference for seven-fold symmetry". Proteins 14 (2): 191–201. doi:10.1002/prot.340140206. PMID 1409568.
- ↑ "The ancient regulatory-protein family of WD-repeat proteins". Nature 371 (6495): 297–300. September 1994. doi:10.1038/371297a0. PMID 8090199.
- ↑ "The WD repeat: a common architecture for diverse functions". Trends Biochem. Sci. 24 (5): 181–5. May 1999. doi:10.1016/S0968-0004(99)01384-5. PMID 10322433.
- ↑ Zhang, Da-Wei (2008). "Structural requirements for PCSK9-mediated degradation of low-density lipoprotein receptor". PNAS 105: 13045–13050. doi:10.1073/pnas.0806312105. PMID 18753623.
- ↑ Betteridge, John (2013). "PCSK9-an exciting target for reducing LDL-cholesterol levels". Nature Reviews Endocrinology 9: 76–78. doi:10.1038/nrendo.2012.254.
- ↑ Chen, Chun-Chi; Cheng, Kuo-Joan; Ko, Tzu-Ping; Guo, Rey-Ting (2015-01-09). "Current Progresses in Phytase Research: Three-Dimensional Structure and Protein Engineering" (in en). ChemBioEng Reviews 2 (2): 76–86. doi:10.1002/cben.201400026. ISSN 2196-9744. https://doi.org/10.1002/cben.201400026.
Further reading
- Branden C, Tooze J. (1999). Introduction to Protein Structure 2nd ed. Garland Publishing: New York, NY.
External links
- SCOP 4-bladed beta propellers
- SCOP 5-bladed beta propellers
- SCOP 6-bladed beta propellers
- SCOP 7-bladed beta propellers
- SCOP 8-bladed beta propellers
