Biology:Interleukin-4 receptor
 Generic protein structure example |
| Interleukin-4 receptor alpha chain, N-terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
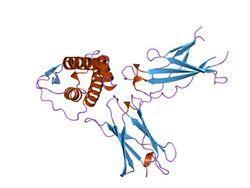 interleukin-4 / receptor alpha chain complex | |||||||||
| Identifiers | |||||||||
| Symbol | IL4Ra_N | ||||||||
| Pfam | PF09238 | ||||||||
| InterPro | IPR015319 | ||||||||
| SCOP2 | 1iar / SCOPe / SUPFAM | ||||||||
| |||||||||
The interleukin 4 receptor is a type I cytokine receptor. It is a heterodimer, that is, composed of two subunits. IL4R is the human gene coding for IL-4Rα, the subunit which combines with either common gamma chain (γc, forming the type I IL4 receptor) or with IL-13Rα1 (forming the type II IL4 receptor).[1]
Function
This gene encodes the alpha chain of the interleukin-4 receptor, a type I transmembrane protein that can bind interleukin 4 and interleukin 13 to regulate IgE antibody production in B cells. Among T cells, the encoded protein also can bind interleukin 4 to promote differentiation of Th2 cells. A soluble form of the encoded protein can be produced by an alternate splice variant or by proteolysis of the membrane-bound protein, and this soluble form can inhibit IL4-mediated cell proliferation and IL5 upregulation by T-cells. Allelic variations in this gene have been associated with atopy, a condition that can manifest itself as allergic rhinitis, sinusitis, asthma, or eczema. Two transcript variants encoding different isoforms, a membrane-bound and a soluble form, have been found for this gene.[2] Interactions of IL-4 with TNFα promote structural changes to vascular endothelial cells, thus playing an important role in tissue inflammation.[3]
The binding of IL-4 or IL-13 to the IL-4 receptor on the surface of macrophages results in the alternative activation of those macrophages. Alternatively activated macrophages (AAMΦ) downregulate inflammatory mediators such as IFNγ during immune responses, particularly with regards to helminth infections.[4]
Interactions
Interleukin-4 receptor has been shown to interact with SHC1.[5][6]
Structure
The N-terminal (extracellular) portion of interleukin-4 receptor is related in overall topology to fibronectin type III modules and folds into a sandwich comprising seven antiparallel beta sheets arranged in a three-strand and a four-strand beta-pleated sheet. They are required for binding of interleukin-4 to the receptor alpha chain, which is a crucial event for the generation of a Th2-dominated early immune response.[7]
See also
- Macrophage-activating factor
- Macrophage polarization
- Cluster of differentiation
- Fibronectin type III domain
References
- ↑ McCormick, Sarah M (14 July 2015). "Commentary: IL-4 and IL-13 receptors and signaling". Cytokine 75 (1): 38–50. doi:10.1016/j.cyto.2015.05.023. PMID 26187331. PMC 4546937. https://doi.org/10.1016/j.cyto.2015.05.023.
- ↑ "Entrez Gene: IL4R interleukin 4 receptor". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=3566.
- ↑ Thornhill, MH; Wellicome, SM; Mahiouz, DL; Lanchbury, JSS; Kyanaung, U; Haskard, DO (Jan 1991). "Tumor-necrosis-factor combines with IL-4 or IFN-gamma to selectively enhance endothelial-cell adhesiveness for T-cells-the contribution of vascular cell-adhesion molecule-1-dependent and molecule-1-independent binding mechanisms". Journal of Immunology 146 (2): 592–598. doi:10.4049/jimmunol.146.2.592. PMID 1702807.
- ↑ "Polarization of host immune responses by helminth-expressed glycans". Ann. N. Y. Acad. Sci. 1253 (1): E1–E13. April 2012. doi:10.1111/j.1749-6632.2012.06618.x. PMID 22974465. Bibcode: 2012NYASA1253E...1T.
- ↑ "Possible involvement of Shc in IL-4-induced germline epsilon transcription in a human B cell line". Biochem. Biophys. Res. Commun. 268 (1): 54–9. February 2000. doi:10.1006/bbrc.2000.2080. PMID 10652211.
- ↑ "Immunoreceptor tyrosine-based inhibitory motif of the IL-4 receptor associates with SH2-containing phosphatases and regulates IL-4-induced proliferation". J. Immunol. 167 (11): 6382–7. December 2001. doi:10.4049/jimmunol.167.11.6382. PMID 11714803.
- ↑ "Crystal structure of the interleukin-4/receptor alpha chain complex reveals a mosaic binding interface". Cell 97 (2): 271–81. April 1999. doi:10.1016/S0092-8674(00)80736-9. PMID 10219247.
Further reading
- "The IL-4 receptor: signaling mechanisms and biologic functions.". Annu. Rev. Immunol. 17 (1): 701–38. 1999. doi:10.1146/annurev.immunol.17.1.701. PMID 10358772. https://zenodo.org/record/1234989.
- "IL-4/IL-13 signaling beyond JAK/STAT.". J. Allergy Clin. Immunol. 105 (6 Pt 1): 1063–70. 2000. doi:10.1067/mai.2000.107604. PMID 10856136.
External links
- CD124+Antigen at the US National Library of Medicine Medical Subject Headings (MeSH)
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Interleukin-4 receptor subunit alpha
 |