Biology:Phosphopentose epimerase
| ribulose-phosphate 3-epimerase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 D-ribulose-5-phosphate 3-epimerase dodekamer, Francisella tularensis | |||||||||
| Identifiers | |||||||||
| EC number | 5.1.3.1 | ||||||||
| CAS number | 9024-20-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Phosphopentose epimerase (also known as ribulose-phosphate 3-epimerase and ribulose 5-phosphate 3-epimerase, EC 5.1.3.1) encoded by the RPE gene is a metalloprotein that catalyzes the interconversion between D-ribulose 5-phosphate and D-xylulose 5-phosphate.[1]
- D-ribulose 5-phosphate [math]\displaystyle{ \rightleftharpoons }[/math] D-xylulose 5-phosphate
This reversible conversion is required for carbon fixation in plants – through the Calvin cycle – and for the nonoxidative phase of the pentose phosphate pathway.[2][3] This enzyme has also been implicated in additional pentose and glucuronate interconversions.
In Cupriavidus metallidurans two copies of the gene coding for PPE are known,[4] one is chromosomally encoded P40117, the other one is on a plasmid Q04539. PPE has been found in a wide range of bacteria, archaebacteria, fungi and plants. All the proteins have from 209 to 241 amino acid residues. The enzyme has a TIM barrel structure.
Nomenclature
The systematic name of this enzyme class is D-ribulose-5-phosphate 3-epimerase. Other names in common use include
- phosphoribulose epimerase,
- erythrose-4-phosphate isomerase,
- phosphoketopentose 3-epimerase,
- xylulose phosphate 3-epimerase,
- phosphoketopentose epimerase,
- ribulose 5-phosphate 3-epimerase,
- D-ribulose phosphate-3-epimerase,
- D-ribulose 5-phosphate epimerase,
- D-ribulose-5-P 3-epimerase,
- D-xylulose-5-phosphate 3-epimerase, and
- pentose-5-phosphate 3-epimerase.
This enzyme participates in 3 metabolic pathways: pentose phosphate pathway, pentose and glucuronate interconversions, and carbon fixation.
The human protein containing this domain is the RPE (gene).
Family
Phosphopentose epimerase belongs to two protein families of increasing hierarchy. This enzyme belongs to the isomerase family, specifically those racemases and epimerases which act on carbohydrates and their derivatives.[1] In addition, the Structural Classification of Proteins database has defined the “ribulose phosphate binding” superfamily for which this epimerase is a member.[1] Other proteins included in this superfamily are 5‘-monophosphate decarboxylase (OMPDC), and 3-keto-l-gulonate 6-phosphate decarboxylase (KGPDC).
Structure
As of late 2007, 4 structures have been solved for this class of enzymes, with PDB accession codes 1H1Y, 1H1Z, 1RPX, and 1TQJ.
Overall
Crystallographic studies have helped elucidate the apoenzyme structure of phosphopentose epimerase. Results of these studies have shown that this enzyme exists as a homodimer in solution.[5][6] Furthermore, Phosphopentose epimerase folds into a (β/α)8 triosephosphate isomerase (TIM) barrel that includes loops.[2] The core barrel is composed of 8 parallel strands that make up the central beta sheet, with helices located in between consecutive strands. The loops in this structure have been known to regulate substrate specificities. Specifically, the loop that connects helix α6 with strand β6 caps the active site upon binding of the substrate.[2]
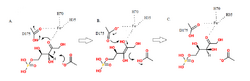
As previously mentioned, Phosphopentose epimerase is a metalloenzyme. It requires a cofactor for functionality and binds one divalent metal cation per subunit.[7] This enzyme has been shown to use Zn2+ predominantly for catalysis, along with Co2+ and Mn2+.[2] However, human phosphopentose epimerase – which is encoded by the RPE gene - differs in that it binds Fe2+ predominantly in catalysis. Fe2+ is octahedrally coordinated and stabilizes the 2,3-enediolate reaction intermediate observed in the figure.[2]
Active site
The β6/α6 loop region interacts with the substrate and regulates access to the active site. Phe147, Gly148, and Ala149 of this region cap the active site once binding has occurred. In addition, the Fe2+ ion is coordinated to His35, His70, Asp37, Asp175, and oxygens O2 and O3 of the substrate.[2] The binding of substrate atoms to the iron cation helps stabilize the complex during catalysis. Mutagenesis studies have also indicated that two aspartic acids are located within the active site and help mediate catalysis through a 1,1-proton transfer reaction.[1] The aspartic acids are the acid/base catalysts. Lastly, once the ligand is attached to the active site, a series of methionines (Met39, Met72, and Met141) restrict further movement through constriction.[8]
Mechanism
Phosphopentose utilizes an acid/base type of catalytic mechanism. The reaction proceeds in such a way that trans-2,3-enediol phosphate is the intermediate.[9][10] The two aspartic acids mentioned above act as proton donors and acceptors. Asp37 and Asp175 are both hydrogen bonded to the iron cation in the active site.[2] When Asp37 is deprotonated, it attacks a proton on the third carbon of D-ribulose 5-phosphate, which forms the intermediate.[11] In a concerted step, as Asp37 grabs a proton, the carbonyl bond on the substrate grabs a second proton from Asp175 to form a hydroxyl group. The iron complex helps stabilize any additional charges. It is C3 of D-ribulose 5-phosphate which undergoes this epimerization, forming D-xylulose 5-phosphate.[8] The mechanism is clearly demonstrated in the figure.
Function
Calvin cycle
Electron microscopy experiments in plants have shown that phosphopentose epimerase localizes to the thylakoid membrane of chloroplasts.[12] This epimerase participates in the third phase of the Calvin cycle, which involves the regeneration of ribulose 1,5-bisphosphate. RuBP is the acceptor of the carbon dioxide (CO2) in the first step of the pathway, which suggests that phosphopentose epimerase regulates flux through the Calvin cycle. Without the regeneration of ribulose 1,5-bisphosphate, the cycle will be unable to continue. Therefore, xylulose 5-phosphate is reversibly converted into ribulose 5-phosphate by this epimerase. Subsequently, phosphoribulose kinase converts ribulose 5-phosphate into ribulose 1,5-bisphosphate.[11]
Pentose phosphate pathway
The reactions of the pentose phosphate pathway (PPP) take place in the cytoplasm. Phosphopentose epimerase specifically affects the nonoxidative portion of the pathway, which involves the production of various sugars and precursors.[2] This enzyme converts ribulose 5-phosphate into the appropriate epimer for the transketolase reaction, xylulose 5-phosphate.[11] Therefore, the reaction that occurs in the pentose phosphate pathway is exactly the reverse of the reaction which occurs in the Calvin cycle. The mechanism remains the same and involves the formation of an enediolate intermediate.
Due to its involvement in this pathway, phosphopentose epimerase is an important enzyme for the cellular response to oxidative stress.[2] The generation of NADPH by the pentose phosphate pathway helps protect cells against reactive oxygen species. NADPH is able to reduce glutathione, which detoxifies the body by producing water from hydrogen peroxide (H2O2).[2] Therefore, not only does phosphopentose epimerase alter flux through the PPP, but it also prevents buildup of peroxides.
Evolution
The structures of many phosphopentose epimerase analogs have been discovered through crystallographic studies.[13][14] Due to its role in the Calvin cycle and the pentose phosphate pathway, the overall structure is conserved. When the sequences of evolutionarily-distant organisms were compared, greater than 50% similarity was observed.[15] However, amino acids positioned at the dimer interface – which are involved in many intermolecular interactions – are not necessarily conserved. It is important to note that the members of the “ribulose phosphate binding” superfamily resulted from divergent evolution from a (β/α)8 - barrel ancestor.[1]
Drug targeting and malaria
The protozoan organism Plasmodium falciparum is a major causative agent of malaria. Phosphopentose epimerase has been implicated in the shikimate pathway, an essential pathway for the propagation of malaria.[16] As the enzyme converts ribulose 5-phosphate into xylulose 5-phosphate, the latter is further metabolized into erythrose 4-phosphate. The shikimate pathway then converts erythrose 4-phosphate into chorismate.[16] It is phosphopentose epimerase which allows Plasmodium falciparum to use erythorse 4-phosphate as a substrate. Due to this enzyme’s involvement in the shikimate pathway, phosphopentose epimerase is a potential drug target for developing antimalarials.
See also
- Phosphopentose Isomerase
- Phosphoribulose Kinase
- Pentose Phosphate Pathway
- TIM barrel
- RPE (human gene encoding Ribulose-phosphate 3-epimerase)
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 "D-Ribulose 5-phosphate 3-epimerase: functional and structural relationships to members of the ribulose-phosphate binding (beta/alpha)8-barrel superfamily". Biochemistry 45 (8): 2493–503. Feb 2006. doi:10.1021/bi052474m. PMID 16489742.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 "Conversion of D-ribulose 5-phosphate to D-xylulose 5-phosphate: new insights from structural and biochemical studies on human RPE". FASEB Journal 25 (2): 497–504. Feb 2011. doi:10.1096/fj.10-171207. PMID 20923965.
- ↑ Mendz, George; Stuart Hazell (1991). "Evidence for a pentose phosphate pathway in Helicobacter pylori". FEMS Microbiology Letters 84 (3): 331–336. doi:10.1111/j.1574-6968.1991.tb04619.x.
- ↑ "The Calvin cycle enzyme pentose-5-phosphate 3-epimerase is encoded within the cfx operons of the chemoautotroph Alcaligenes eutrophus". Journal of Bacteriology 174 (22): 7337–44. Nov 1992. doi:10.1128/jb.174.22.7337-7344.1992. PMID 1429456.
- ↑ "D-Ribulose-5-phosphate 3-epimerase: cloning and heterologous expression of the spinach gene, and purification and characterization of the recombinant enzyme". Plant Physiology 118 (1): 199–207. Sep 1998. doi:10.1104/pp.118.1.199. PMID 9733539.
- ↑ "Purification, properties and assay of D-ribulose 5-phosphate 3-epimerase from human erythrocytes". The Biochemical Journal 211 (3): 617–23. Jun 1983. doi:10.1042/bj2110617. PMID 6882362.
- ↑ "Ribulose-phosphate 3-epimerase". UniProt. https://www.uniprot.org/uniprot/Q96AT9.
- ↑ Jump up to: 8.0 8.1 "Structure and catalytic mechanism of the cytosolic D-ribulose-5-phosphate 3-epimerase from rice". Journal of Molecular Biology 326 (1): 127–35. Feb 2003. doi:10.1016/S0022-2836(02)01374-8. PMID 12547196.
- ↑ Das, Debajoyti (1978). Biochemistry. Academic Publishers. pp. 454–460.
- ↑ "On the mechanism of the pentose phosphate epimerases". The Journal of Biological Chemistry 247 (18): 5862–6. Sep 1972. doi:10.1016/S0021-9258(19)44837-0. PMID 4560420. http://www.jbc.org/content/247/18/5862.
- ↑ Jump up to: 11.0 11.1 11.2 Berg, Jeremy (2006). Biochemistry. WH Freeman and Company. pp. 570–580. ISBN 978-0-7167-8724-2.
- ↑ "Identification of a catalytic aspartyl residue of D-ribulose 5-phosphate 3-epimerase by site-directed mutagenesis". The Journal of Biological Chemistry 274 (4): 2132–6. Jan 1999. doi:10.1074/jbc.274.4.2132. PMID 9890975.
- ↑ "Cloning of the amphibolic Calvin cycle/OPPP enzyme D-ribulose-5-phosphate 3-epimerase (EC 5.1.3.1) from spinach chloroplasts: functional and evolutionary aspects". Plant Molecular Biology 29 (6): 1279–91. Dec 1995. doi:10.1007/bf00020468. PMID 8616224.
- ↑ "Structure of D-ribulose 5-phosphate 3-epimerase from Synechocystis to 1.6 A resolution". Acta Crystallographica Section D 60 (Pt 9): 1687–90. Sep 2004. doi:10.1107/S0907444904015896. PMID 15333955.
- ↑ "Chloroplast pentose-5-phosphate 3-epimerase from potato: cloning, cDNA sequence, and tissue-specific enzyme accumulation". FEBS Letters 377 (3): 349–52. Dec 1995. doi:10.1016/0014-5793(95)01373-3. PMID 8549753.
- ↑ Jump up to: 16.0 16.1 "Structure of a ribulose 5-phosphate 3-epimerase from Plasmodium falciparum". Proteins 62 (2): 338–42. Feb 2006. doi:10.1002/prot.20764. PMID 16304640.
External links
 |