Chemistry:1-Aminopropan-2-ol
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Aminopropan-2-ol | |
| Other names
1-Amino-2-propanol
Isopropanolamine MIPA; Threamine | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H9NO | |
| Molar mass | 75.111 g·mol−1 |
| Appearance | liquid |
| Odor | ammonia-like |
| Density | 0.973 g/mL (18 °C) [1] |
| Melting point | 1.74 °C (35.13 °F; 274.89 K) |
| Boiling point | 159.46 °C (319.03 °F; 432.61 K) |
| soluble | |
| Solubility | soluble in alcohol, ether, acetone, benzene, CCl4 |
Refractive index (nD)
|
1.4479 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 77 °C (171 °F; 350 K) |
| 374 °C (705 °F; 647 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4.26 g/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
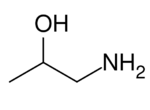
1-Aminopropan-2-ol is the organic compound with the formula CH
3CH(OH)CH
2NH
2. It is an amino alcohol. The term isopropanolamine may also refer more generally to the additional homologs diisopropanolamine (DIPA) and triisopropanolamine (TIPA).
1-Aminopropan-2-ol is chiral. It can be prepared by the addition of aqueous ammonia to propylene oxide.[2]
Biosynthesis
(R)-1-Aminopropan-2-ol is one of the components incorporated in the biosynthesis of cobalamin. The O-phosphate ester is produced from threonine by the enzyme Threonine-phosphate decarboxylase.[3][4]
Applications
The isopropanolamines are used as buffers. They are good solubilizers of oil and fat, so they are used to neutralize fatty acids and sulfonic acid-based surfactants. Racemic 1-aminopropan-2-ol is typically used in metalworking fluid, waterborne coatings, personal care products, and in the production of titanium dioxide and polyurethanes.[5] It is an intermediate in the synthesis of a variety of pharmaceutical drugs.[citation needed]
(R)-1-aminopropan-2-ol is metabolised to aminoacetone by the enzyme (R)-aminopropanol dehydrogenase.[6]
Synthesis of Hexylcaine is one application.
References
- ↑ Amino-2-propanol at Sigma-Aldrich
- ↑ Smith, Michael B. (19 February 2020). March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. John Wiley & Sons. ISBN 9781119371809.
- ↑ Cheong, Cheom-Gil; Bauer, Cary B.; Brushaber, Kevin R.; Escalante-Semerena, Jorge C.; Rayment, Ivan (2002). "Three-Dimensional Structure of the L-Threonine-O-3-phosphate Decarboxylase (CobD) Enzyme from Salmonella enterica". Biochemistry 41 (15): 4798–4808. doi:10.1021/bi012111w. PMID 11939774.
- ↑ Warren, Martin J.; Raux, Evelyne; Schubert, Heidi L.; Escalante-Semerena, Jorge C. (2002). "The biosynthesis of adenosylcobalamin (Vitamin B12)". Natural Product Reports 19 (4): 390–412. doi:10.1039/b108967f. PMID 12195810.
- ↑ "Monoisopropanolamine". Nanjing HBL International. http://www.hbltrade.com/pid10029823/Monoisopropanolamine.htm.
- ↑ Turner, JM (1967). "Microbial metabolism of amino ketones. L-1-aminopropan-2-ol dehydrogenase and L-threonine dehydrogenase in Escherichia coli". Biochemical Journal 104 (1): 112–121. doi:10.1042/bj1040112. PMID 5340733.
 |

