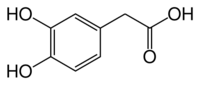
Chemistry:3,4-Dihydroxyphenylacetic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3,4-Dihydroxyphenyl)acetic acid | |
| Other names
2-(3,4-Dihydroxyphenyl)acetic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | 3,4-Dihydroxyphenylacetic+Acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H8O4 | |
| Molar mass | 168.148 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
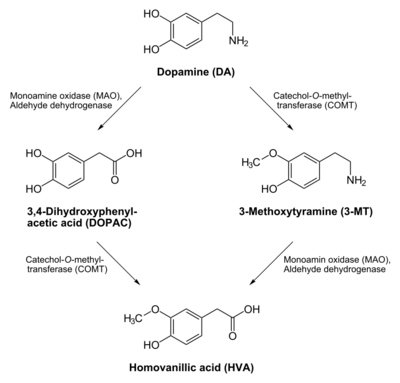
3,4-Dihydroxyphenylacetic acid (DOPAC) is a metabolite of the neurotransmitter dopamine. Dopamine can be metabolized into one of three substances. One such substance is DOPAC. Another is 3-methoxytyramine (3-MT). Both of these substances are degraded to form homovanillic acid (HVA). Both degradations involve the enzymes monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT), albeit in reverse order: MAO catalyzes dopamine to DOPAC, and COMT catalyzes DOPAC to HVA; whereas COMT catalyzes dopamine to 3-MT and MAO catalyzes 3-MT to HVA. The third metabolic end-product of dopamine is norepinephrine (noradrenaline).
DOPAC can be oxidized by hydrogen peroxide, leading to the formation of toxic metabolites which destroy dopamine storage vesicles in the substantia nigra. This may contribute to the failure of levodopa treatment of Parkinson's disease. A MAO-B inhibitor such as selegiline or rasagiline can prevent this from happening.[citation needed]
It can also be found in the bark of Eucalyptus globulus.[1]
This product has been synthesized (52% yield) from 4-hydroxyphenylacetic acid via aerobic biotransformation using whole cell cultures of Arthrobacter protophormiae.[2][3]
References
- ↑ Santos, Sónia A. O.; Freire, Carmen S. R.; Domingues, M. Rosário M.; Silvestre, Armando J. D.; Neto, Carlos Pascoal (2011). "Characterization of Phenolic Components in Polar Extracts of Eucalyptus globulus Labill. Bark by High-Performance Liquid Chromatography–Mass Spectrometry". Journal of Agricultural and Food Chemistry 59 (17): 9386–93. doi:10.1021/jf201801q. PMID 21761864.
- ↑ Robins, Karen T.; Osorio-Lozada, Antonio; Avi, Manuela; Meyer, Hans-Peter (2009). "Lonza: Biotechnology – A Key Ingredient for Success in the Future". CHIMIA International Journal for Chemistry 63 (6): 327–330. doi:10.2533/chimia.2009.327.
- ↑ Sutton, Peter; Whittall, John (2012). Practical Methods for Biocatalysis and Biotransformations 2. Chichester, West Sussex: John Wiley & Sons, Ltd.. pp. 150–153. ISBN 9781119991397. https://books.google.com/books?id=WlODZ-WX8vIC&q=9781119991397.
{{Navbox
| name = Neurotransmitter metabolism intermediates | title = Neurotransmitter metabolic intermediates | state = autocollapse| | listclass = hlist
| group1 = catecholamines | list1 = {{Navbox|child
| group1 = Anabolism
(tyrosine→epinephrine) | list1 =
- Tyrosine → Levodopa → [[Chemistry:Dop[[Chemistry:Dopamine → Norepinephrine|Norepinephrine]] → Epinephrine
| group2 = Catabolism/
metabolites
| list2 =
| dopamine: | |
|---|---|
| norepinephrine: | |
| epinephrine: |
}}
| group3 = tryptophan→serotonin| list3 =
| anabolism: | |
|---|---|
| catabolism: |