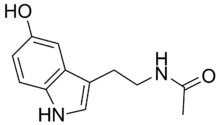
Chemistry:N-Acetylserotonin
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-[2-(5-Hydroxy-1H-indol-3-yl)ethyl]acetamide | |
| Other names
N-Acetyl-5-hydroxytryptamine
N-Acetyl-5-HT | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| MeSH | N-Acetylserotonin N-Acetylserotonin |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H14N2O2 | |
| Molar mass | 218.256 g·mol−1 |
| Density | 1.268 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Acetylserotonin (NAS), also known as normelatonin, is a naturally occurring chemical intermediate in the endogenous production of melatonin from serotonin.[1][2] It also has biological activity in its own right, including acting as a melatonin receptor agonist, an agonist of the TrkB, and having antioxidant effects.
Biological function
Like melatonin, NAS is an agonist at the melatonin receptors MT1, MT2, and MT3, and may be considered to be a neurotransmitter.[3][4][5][6] In addition, NAS is distributed in some areas of the brain where serotonin and melatonin are not, suggesting that it may have unique central duties of its own instead of merely functioning as a precursor in the synthesis of melatonin.[3] NAS is known to have anti-depressant, neurotrophic and cognition-enhancing effects [7][8] and has been proposed to be a target for the treatment of aging-associated cognitive decline and depression [8]
TrkB receptor
NAS has been shown to act as a potent TrkB receptor agonist, while serotonin and melatonin do not.[3] Subchronic and chronic administration of NAS to adult mice induces proliferation of neural progenitor cells (NPC)s, blockage of TrkB abolished this effect suggesting that it is TrkB-dependent.[9] NAS was also found to significantly enhance NPC proliferation in sleep-deprived mice.[9] It is thought that the anti-depressant and neurotrophic effects of NAS are in part due to its role as a TrkB agonist.[7]
Antioxidant properties
NAS acts as a potent antioxidant, NAS effectiveness as an anti-oxidant has been found to be different depending on the experimental model used, it has been described as being between 5 and 20 times more effect than melatonin at protecting against oxidant damage.[10] NAS has been shown to protect against lipid peroxidation in microsomes and mitochondria.[11] NAS has also been reported to lower resting levels of ROS in peripheral blood lymphocytes and to exhibit anti-oxidant effects against t-butylated hydroperoxide- and diamide-induced ROS.[12] NAS has also been observed to inhibit nitric oxide synthase.[13]
Anti-inflammatory effects
NAS has been reported to have anti-inflammatory effects. NAS has been shown to inhibit LPS-stimulated production of the proinflammatory cytokine TNF-alpha in differentiated THP-1-derived human monocytes.[14]
Miscellaneous
NAS may play a role in the antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) and monoamine oxidase inhibitors (MAOIs).[3] The SSRI fluoxetine and the MAO-A inhibitor clorgyline upregulate AANAT indirectly through serotonergic mechanisms and thereby increase NAS levels after chronic administration, and this correlates with the onset of their antidepressant effects.[3][15] Furthermore, light exposure inhibits the synthesis of NAS and reduces the antidepressant effects of MAOIs.[3] In addition, AANAT knockout mice display significantly greater immobility times versus control mice in animal models of depression.[3] These data support a potential role for NAS in mood regulation and in antidepressant-induced therapeutic benefits.
Through a currently unidentified mechanism, NAS may be the cause of the orthostatic hypotension seen with clinical treatment of MAOIs.[15][16] It reduces blood pressure in rodents, and pinealectomy (the pineal gland being a major site of NAS and melatonin synthesis) abolishes the hypotensive effects of clorgyline.[15][16]
Biochemistry
NAS is produced from serotonin by the enzyme aralkylamine N-acetyltransferase (AANAT) and is converted to melatonin by acetylserotonin O-methyltransferase (ASMT).
NAS is able to penetrate the blood–brain barrier, unlike serotonin.[17]
See also
References
- ↑ "Enzymatic O-methylation of N-acetylserotonin to melatonin". Science 131 (3409): 1312. April 1960. doi:10.1126/science.131.3409.1312. PMID 13795316. Bibcode: 1960Sci...131.1312A.
- ↑ "Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin". Biochimica et Biophysica Acta 43: 352–3. September 1960. doi:10.1016/0006-3002(60)90453-4. PMID 13784117.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "N-acetylserotonin activates TrkB receptor in a circadian rhythm". Proceedings of the National Academy of Sciences of the United States of America 107 (8): 3876–81. February 2010. doi:10.1073/pnas.0912531107. PMID 20133677. Bibcode: 2010PNAS..107.3876J.
- ↑ "Pharmacological characterization, molecular subtyping, and autoradiographic localization of putative melatonin receptors in uterine endometrium of estrous rats". Life Sciences 66 (17): 1581–91. March 2000. doi:10.1016/S0024-3205(00)00478-1. PMID 11261588.
- ↑ "Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists". British Journal of Pharmacology 127 (5): 1288–94. July 1999. doi:10.1038/sj.bjp.0702658. PMID 10455277.
- ↑ "Characterization of 2-[125Iiodomelatonin binding sites in Syrian hamster peripheral organs"]. The Journal of Pharmacology and Experimental Therapeutics 290 (1): 334–40. July 1999. PMID 10381796. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=10381796.
- ↑ 7.0 7.1 Tosini G., Ye K. & Iuvone PM (2012). "Neuroprotection, neurogenesis, and the sleepy brain". Neuroscientist 18 (6): 645–653. doi:10.1177/1073858412446634. PMID 22585341.
- ↑ 8.0 8.1 Oxenkrug G. & Ratner R. (2012). "N-acetylserotonin and aging-associated cognitive impairment and depression". Aging Dis 3 (4): 330–338. PMID 23185714.
- ↑ 9.0 9.1 Sompol P.; Liu X.; Baba K.; Paul KN.; Tosini G.; Iuvone PM.; Ye K. (2011). "N-acetylserotonin promotes hippocampal neuroprogenitor cell proliferation in sleep-deprived mice". Proc. Natl. Acad. Sci. U.S.A. 108 (21): 8844–9. doi:10.1073/pnas.1105114108. PMID 21555574. Bibcode: 2011PNAS..108.8844S.
- ↑ Oxenkrug G (2005). "Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications". Ann. N. Y. Acad. Sci. 1053: 334–47. doi:10.1111/j.1749-6632.2005.tb00042.x. PMID 16179540.
- ↑ Gavazza MB.; Català A. (2004). "Protective effect of N-acetyl-serotonin on the nonenzymatic lipid peroxidation in rat testicular microsomes and mitochondria". J. Pineal Res. 37 (3): 153–60. doi:10.1111/j.1600-079x.2004.00150.x. PMID 15357659.
- ↑ Wölfler A.; Abuja PM.; Schauenstein K.; Liebmann PM. (1999). "N-acetylserotonin is a better extra- and intracellular antioxidant than melatonin". FEBS Lett 449 (2–3): 206–10. doi:10.1016/s0014-5793(99)00435-4. PMID 10338133.
- ↑ Peter Klemm; Markus Hecker; Hannelore Stockhausen; Chin Chen Wu; Christoph Thiemermann (Aug 1995). "Inhibition by N-acetyl-5-hydroxytryptamine of nitric oxide synthase expression in cultured cells and in the anaesthetized rat.". Br J Pharmacol 115 (7): 1175–1181. doi:10.1111/j.1476-5381.1995.tb15021.x. PMID 7582541.
- ↑ Perianayagam MC.; Oxenkrug GF.; Jaber BL. (2005). "Immune-modulating effects of melatonin, N-acetylserotonin, and N-acetyldopamine". Ann. N. Y. Acad. Sci. 1053: 386–93. doi:10.1111/j.1749-6632.2005.tb00046.x. PMID 16179544.
- ↑ 15.0 15.1 15.2 Oxenkrug GF (1999). "Antidepressive and antihypertensive effects of MAO-A inhibition: role of N-acetylserotonin. A review". Neurobiology (Budapest, Hungary) 7 (2): 213–24. PMID 10591054.
- ↑ 16.0 16.1 Oxenkrug GF (1997). "[N-acetylserotonin and hypotensive effect of MAO-A inhibitors]" (in ru). Voprosy Meditsinskoi Khimii 43 (6): 522–6. PMID 9503569.
- ↑ "N-Acetyl Serotonin". DrugBank. http://www.drugbank.ca/drugs/DB04275.
{{Navbox
| name = Neurotransmitter metabolism intermediates | title = Neurotransmitter metabolic intermediates | state = autocollapse| | listclass = hlist
| group1 = catecholamines | list1 = {{Navbox|child
| group1 = Anabolism
(tyrosine→epinephrine) | list1 =
- Tyrosine → Levodopa → [[Chemistry:Dop[[Chemistry:Dopamine → Norepinephrine|Norepinephrine]] → Epinephrine
| group2 = Catabolism/
metabolites
| list2 =
| dopamine: | |
|---|---|
| norepinephrine: | |
| epinephrine: |
}}
| group3 = tryptophan→serotonin| list3 =
| anabolism: | |
|---|---|
| catabolism: |


