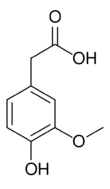
Chemistry:Homovanillic acid
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(4-Hydroxy-3-methoxyphenyl)acetic acid | |||
| Other names
2-(4-Hydroxy-3-methoxyphenyl)acetic acid; 3-Methoxy-4-hydroxyphenylacetic acid; 4-Hydroxy-3-methoxy-benzeneacetic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
| MeSH | Homovanillic+acid | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C9H10O4 | |||
| Molar mass | 182.175 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Homovanillic acid (HVA) is a major catecholamine metabolite that is produced by a consecutive action of monoamine oxidase and catechol-O-methyltransferase on dopamine.[1] Homovanillic acid is used as a reagent to detect oxidative enzymes, and is associated with dopamine levels in the brain.
In psychiatry and neuroscience, brain and cerebrospinal fluid levels of HVA are measured as a marker of metabolic stress caused by 2-deoxy-D-glucose.[2] HVA presence supports a diagnosis of neuroblastoma and malignant pheochromocytoma.
Fasting plasma levels of HVA are known to be higher in females than in males.[citation needed] This does not seem to be influenced by adult hormonal changes, as the pattern is retained in the elderly and post-menopausal as well as transgender people according to their genetic sex, both before and during cross-sex hormone administration.[3] Differences in HVA have also been correlated to tobacco usage, with smokers showing significantly lower amounts of plasma HVA.
See also
References
- ↑ Lambert, G.W.; Eisenhofer, G.; Jennings, G.L.; Esler, M.D. (1993). "Regional homovanillic acid production in humans". Life Sciences 53 (1): 63–75. doi:10.1016/0024-3205(93)90612-7. PMID 8515683.
- ↑ "Evidence that brain tissue volumes are associated with HVA reactivity to metabolic stress in schizophrenia". Schizophr. Res. 86 (1–3): 45–53. September 2006. doi:10.1016/j.schres.2006.05.001. PMID 16806836.
- ↑ "The sex difference of plasma homovanillic acid is unaffected by cross-sex hormone administration in transgender people". J Endocrinol 187 (1): 109–16. July 2005. doi:10.1677/joe.1.06307. PMID 16214946. http://joe.endocrinology-journals.org/cgi/content/abstract/187/1/109. Retrieved 2011-01-30.
{{Navbox
| name = Neurotransmitter metabolism intermediates | title = Neurotransmitter metabolic intermediates | state = autocollapse| | listclass = hlist
| group1 = catecholamines | list1 = {{Navbox|child
| group1 = Anabolism
(tyrosine→epinephrine) | list1 =
- Tyrosine → Levodopa → [[Chemistry:Dop[[Chemistry:Dopamine → Norepinephrine|Norepinephrine]] → Epinephrine
| group2 = Catabolism/
metabolites
| list2 =
| dopamine: | |
|---|---|
| norepinephrine: | |
| epinephrine: |
}}
| group3 = tryptophan→serotonin| list3 =
| anabolism: | |
|---|---|
| catabolism: |