Chemistry:Avatrombopag
From HandWiki
Short description: Chemical compound
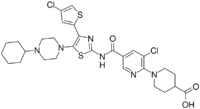 | |
| Clinical data | |
|---|---|
| Pronunciation | a" va trom' boe pag |
| Trade names | Doptelet |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618032 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C29H34Cl2N6O3S2 |
| Molar mass | 649.65 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Avatrombopag, sold under the brand name Doptelet, is a medication that used for certain conditions that lead to thrombocytopenia (low platelets) such as thrombocytopenia associated with chronic liver disease in adults who are to undergo a planned medical or dental procedure.[4][5] It was approved for medical use in the United States in May 2018,[6][7] the European Union in June 2019,[8] and Australia in January 2023.[1]
It acts as a thrombopoietin receptor agonist.[9]
References
- ↑ 1.0 1.1 1.2 "Doptelet". 30 January 2023. https://www.tga.gov.au/resources/auspmd/doptelet.
- ↑ "Doptelet (Swedish Orphan Biovitrum Pty Ltd)". 16 February 2023. https://www.tga.gov.au/resources/prescription-medicines-registrations/doptelet-swedish-orphan-biovitrum-pty-ltd.
- ↑ "Notice: Multiple additions to the Prescription Drug List (PDL) [2023-12-22"]. 22 December 2023. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/multiple-additions-2023-12-22.html.
- ↑ "Current Advance in Thrombopoietin Receptor Agonists in the Management of Thrombocytopenia Associated With Chronic Liver Disease: Focus on Avatrombopag". Clinical Medicine Insights. Blood Disorders 12: 1179545X19875105. 2019. doi:10.1177/1179545X19875105. PMID 31673229.
- ↑ "Avatrombopag: A Review in Thrombocytopenia". Drugs 81 (16): 1905–1913. November 2021. doi:10.1007/s40265-021-01613-y. PMID 34709601.
- ↑ "FDA approves new drug for patients with chronic liver disease who have low blood platelets and are undergoing a medical procedure". U.S. Food and Drug Administration (FDA) (Press release). 21 May 2018. Retrieved 2 May 2020.
- ↑ "Drug Approval Package: Doptelet (avatrombopag)". 28 June 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210238Orig1s000TOC.cfm.
- ↑ "Doptelet EPAR". 24 April 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/doptelet.
- ↑ "The structure, function, and clinical use of the thrombopoietin receptor agonist avatrombopag". Blood Reviews 53: 100909. November 2021. doi:10.1016/j.blre.2021.100909. PMID 34815110.
 |
