Chemistry:Tranexamic acid
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | \ˌtran-eks-ˌam-ik-\ |
| Trade names | Cyklokapron, others |
| Other names | TXA |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612021 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 34% |
| Elimination half-life | 3.1 h |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
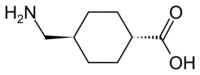
| Formula | C8H15NO2 |
| Molar mass | 157.213 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tranexamic acid is a medication used to treat or prevent excessive blood loss from major trauma, postpartum bleeding, surgery, tooth removal, nosebleeds, and heavy menstruation.[7][8] It is also used for hereditary angioedema.[7][3] It is taken either by mouth, injection into a vein,[7] or by intramuscular injection.
Tranexamic acid is a synthetic analog of the amino acid lysine. It serves as an antifibrinolytic by reversibly binding four to five lysine receptor sites on plasminogen. This decreases the conversion of plasminogen to plasmin, preventing fibrin degradation and preserving the framework of fibrin's matrix structure.[5] Tranexamic acid has roughly eight times the antifibrinolytic activity of an older analogue, ε-aminocaproic acid. Tranexamic acid also directly inhibits the activity of plasmin with weak potency (IC50 = 87 mM),[9] and it can block the active-site of urokinase plasminogen activator (uPA) with high specificity (Ki = 2 mM), one of the highest among all the serine proteases.[10]
Side effects are rare;[3] they include changes in color vision, seizures, blood clots, and allergic reactions.[3] Tranexamic acid appears to be safe for use during pregnancy and breastfeeding.[3][11] Tranexamic acid is an antifibrinolytic medication.[12]
Tranexamic acid was first made in 1962 by Japanese researchers Shosuke and Utako Okamoto.[13] It is on the World Health Organization's List of Essential Medicines.[14] Tranexamic acid is available as a generic drug.[15]
Uses
Medical uses
Tranexamic acid is frequently used following major trauma.[16] Tranexamic acid is used to prevent and treat blood loss in a variety of situations, such as dental procedures, heavy menstrual bleeding, and surgeries with high risk of blood loss.[17][18]
Trauma
Tranexamic acid has been found to decrease the risk of death due to any cause in people who have significant bleeding due to trauma.[19][20][21][22] It is most effective if taken within the first three hours following major trauma.[23] It also decreases the risk of death if given within the first three hours of brain injury.[24]
Menstrual bleeding
Tranexamic acid is sometimes used to treat heavy menstrual bleeding.[18] When taken by mouth it both safely and effectively treats regularly occurring heavy menstrual bleeding and improves quality of life.[5][25][26] Another study demonstrated that the dose does not need to be adjusted in females who are between ages 12 and 16.[5] In a 10-year study, tranexamic acid and other oral medicines (mefenamic acid) were found to be as effective as the levonorgestrel intrauterine coil; the same proportion of women had not had surgery for heavy bleeding and had similar improvements in their quality of life.[27][28]
Childbirth
Tranexamic acid is sometimes used (often in conjunction with oxytocin) to reduce bleeding after childbirth.[29] Death due to postpartum bleeding is reduced in women receiving tranexamic acid.[8]
Surgery
- Tranexamic acid is sometimes used in orthopedic surgery to reduce blood loss, to the extent of reducing or altogether abolishing the need for perioperative blood transfusion. It is of proven value in clearing the field of surgery and reducing blood loss when given before or after surgery. Drain and number of transfusions are reduced.[30][31][32]
- In surgical corrections of craniosynostosis in children it reduces the need for blood transfusions.[33]
- In spinal surgery (e.g., scoliosis), correction with posterior spinal fusion using instrumentation, to prevent excessive blood loss.[34][35]
- In cardiac surgery, both with and without cardiopulmonary bypass (e.g., coronary artery bypass surgery), it is used to prevent excessive blood loss.[30]
Dentistry
In the United States, tranexamic acid is FDA-approved for short-term use in people with severe bleeding disorders who are about to have dental surgery.[36] Tranexamic acid is used for a short period before and after the surgery to prevent major blood loss and decrease the need for blood transfusions.[37]
Tranexamic acid is used in dentistry in the form of a 5% mouth rinse after extractions or surgery in patients with prolonged bleeding time; e.g., from acquired or inherited disorders.[38]
In China, tranexamic acid is allowed in over-the-counter toothpaste, with six products using the drug. As of 2018[update], there are no limits on dosage, nor requirements for labeling the concentration.[39] 0.05% TXA in toothpaste is allowed OTC in Hong Kong.[40] <5% TXA in over-the-counter toothpaste is first patented and marketed by Lion Corporation in Japan,[41] where it is still sold.[42] Presence of unauthorized TXA has led to the Canadian recall of a Yunnan Baiyao toothpaste in 2019.[43]
Hematology
There is not enough evidence to support the routine use of tranexamic acid to prevent bleeding in people with blood cancers.[44] However, several trials are currently assessing this use of tranexamic acid.[44] For people with inherited bleeding disorders (e.g. von Willebrand's disease), tranexamic acid is often given.[45] It has also been recommended for people with acquired bleeding disorders (e.g., directly acting oral anticoagulants (DOACs)) to treat serious bleeding.[46]
Nosebleeds
The use of tranexamic acid, applied directly to the area that is bleeding or taken by mouth, appears useful to treat nose bleeding compared to packing the nose with cotton pledgets alone.[47][48][49] It decreases the risk of rebleeding within 10 days.[50]
Cosmetic uses
Tranexamic acid can be used in skincare products as a cosmetic active to reduce the appearance of inflammation and hyperpigmentation. Tranexamic acid is a zwitterion amino acid, and has a low permeability coefficient in the stratum corneum.[51] Tranexamic acid can be combined with penetration enhancers and microneedling to overcome this limitation.[52] Cosmetic uses may also employ lipophilic derivatives of tranexamic acid (ester prodrugs like Cetyl tranexamate mesylate) that are not zwitterionic and thus have improved skin permeability.[53][54][55]
Contraindications
- Allergic to tranexamic acid
- History of seizures
- History of venous or arterial thromboembolism or active thromboembolic disease
- Severe kidney impairment due to accumulation of the medication, dose adjustment is required in mild or moderate kidney impairment[7]
Adverse effects
Side effects are rare.[3] Reported adverse events include seizures, changes in color vision, blood clots, and allergic reactions such as anaphylaxis.[3] Whether the risk of venous thromboembolism (blood clots) is increased is a matter of debate. The risk is mentioned in the product literature,[5] and they were reported in post marketing experience.[5] Despite this, and the inhibitory effect of tranexamic acid on blood clot breakdown, large studies of the use of tranexamic acid have not shown an increase in the risk of venous or arterial thrombosis,[56][57] even in people who had previously experienced thrombosis under other circumstances.[57]
Special populations
- For pregnancy, no harm has been found in animal studies.[5]
- Small amounts appear in breast milk if taken during lactation.[5][58]
Society and culture
Tranexamic acid was first synthesized in 1962 by Japanese researchers Shosuke and Utako Okamoto.[13] It is on the World Health Organization's List of Essential Medicines.[14]
Brand names
Tranexamic acid is marketed in the US and Australia in tablet form as Lysteda[5] and in Australia, Sweden[59] and Jordan it is marketed in an IV form and tablet form as Cyklokapron, in the UK and Sweden[59] as Cyclo-F. In the UK it is also marketed as Femstrual, in Asia as Transcam, in Bangladesh as Intrax & Tracid, in India as Pause, in Pakistan as Transamin, in Indonesia as Kalnex, in South America as Espercil, in Japan as Nicolda, in France, Poland, Belgium, and Romania as Exacyl and in Egypt as Kapron. In the Philippines, its capsule form is marketed as Hemostan and in Israel as Hexakapron.
Legal status
The US Food and Drug Administration (FDA) approved tranexamic acid oral tablets (brand name Lysteda) for the treatment of heavy menstrual bleeding in November 2009.[5][60][61]
In March 2011, the status of tranexamic acid for the treatment of heavy menstrual bleeding was changed in the UK, from POM (Prescription only Medicines) to P (Pharmacy Medicines)[62] and became available over the counter in UK pharmacies under the brand names of Cyklo-F and Femstrual.[63]
Research
Tranexamic acid might alleviate neuroinflammation in some experimental settings.[64]
Tranexamic acid can be used in case of postpartum hemorrhage; it can decrease the risk of death due to bleeding by one third according to the WHO.[65]
Tentative evidence supports the use of tranexamic acid in hemoptysis.[66][67]
In hereditary hemorrhagic telangiectasia: tranexamic acid has been shown to reduce the frequency of epistaxis in patients with severe and frequent nosebleed episodes from hereditary hemorrhagic telangiectasia.[69]
In melasma: tranexamic acid is sometimes used in skin whitening as a topical agent, injected into a lesion, or taken by mouth, both alone and as an adjunct to laser therapy; as of 2017 its safety seemed reasonable but its efficacy for this purpose was uncertain because there had been no large scale randomized controlled studies nor long term follow-up studies.[70][71] It is allowed as a quasi-drug for skin whitening in Japan.[72]
In hyphema: tranexamic acid is effective in reducing the risk of secondary hemorrhage outcomes in people with traumatic hyphema.[73]
In liver resection: tranexamic acid did not reduce bleeding or transfusions but did increase complications.[74]
References
- ↑ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". 21 June 2022. https://www.tga.gov.au/resources/publication/publications/prescription-medicines-registration-new-generic-medicines-and-biosimilar-medicines-2017.
- ↑ "TRANEXAMIC ACID WAYMADE (Waymade Australia Pty Limited) | Therapeutic Goods Administration (TGA)". https://www.tga.gov.au/resources/prescription-medicines-registrations/tranexamic-acid-waymade-waymade-australia-pty-limited.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Cyklokapron Tablets - Summary of Product Characteristics (SPC) - (eMC)". September 2016. https://www.medicines.org.uk/emc/medicine/16512.
- ↑ "Evana Heavy Period Relief Summary of Product Characteristics (SmPC)". 24 April 2024. https://www.medicines.org.uk/emc/product/15636/smpc.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 "Lysteda- tranexamic acid tablet". 2 December 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a7e3180d-cf5b-4c2c-a31f-68bf6181f19a.
- ↑ "会議事録" (in Japanese). 薬事・食品衛生審議会一般用医薬品部会. 22 March 2007. https://www.mhlw.go.jp/content/shingi___2007___03___txt___s0322-6.txt. Retrieved 7 September 2022.
- ↑ 7.0 7.1 7.2 7.3 British national formulary: BNF 69 (69 ed.). British Medical Association. 2015. p. 170. ISBN 978-0-85711-156-2.
- ↑ 8.0 8.1 "Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial". Lancet 389 (10084): 2105–2116. 2017. doi:10.1016/S0140-6736(17)30638-4. PMID 28456509.
- ↑ "X-ray crystal structure of plasmin with tranexamic acid-derived active site inhibitors". Blood Advances 1 (12): 766–771. 2017. doi:10.1182/bloodadvances.2016004150. PMID 29296720.
- ↑ "Tranexamic acid is an active site inhibitor of urokinase plasminogen activator". Blood Advances 3 (5): 729–733. 2019. doi:10.1182/bloodadvances.2018025429. PMID 30814058.
- ↑ "Tranexamic acid Use During Pregnancy". https://www.drugs.com/pregnancy/tranexamic-acid.html.
- ↑ "Tranexamic Acid Injection - FDA prescribing information, side effects and uses". https://www.drugs.com/pro/tranexamic-acid-injection.html.
- ↑ 13.0 13.1 "Utako Okamoto". Lancet 387 (10035): 2286. 2016. doi:10.1016/S0140-6736(16)30697-3. PMID 27308678.
- ↑ 14.0 14.1 The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. WHO/MHP/HPS/EML/2023.02.
- ↑ Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2015. p. 415. ISBN 978-1-284-05756-0.
- ↑ "CRASH-2 Study of Tranexamic Acid to Treat Bleeding in Trauma Patients: A Controversy Fueled by Science and Social Media". Journal of Blood Transfusion 2015. 2015. doi:10.1155/2015/874920. PMID 26448897.
- ↑ "Tranexamic Acid in Hip and Knee Arthroplasty". The Journal of the American Academy of Orthopaedic Surgeons 23 (12): 732–40. 2015. doi:10.5435/JAAOS-D-14-00223. PMID 26493971.
- ↑ 18.0 18.1 "Tranexamic acid--an old drug still going strong and making a revival". Thrombosis Research 135 (2): 231–42. 2015. doi:10.1016/j.thromres.2014.11.012. PMID 25559460.
- ↑ "The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients". Health Technology Assessment 17 (10): 1–79. March 2013. doi:10.3310/hta17100. PMID 23477634.
- ↑ "Antifibrinolytic drugs for acute traumatic injury". The Cochrane Database of Systematic Reviews 2015 (5). May 2015. doi:10.1002/14651858.CD004896.pub4. PMID 25956410.
- ↑ "Traumatic hemorrhagic shock: advances in fluid management". Emergency Medicine Practice 13 (11): 1–19; quiz 19–20. November 2011. PMID 22164397. http://www.ebmedicine.net/store.php?paction=showProduct&catid=8&pid=244.
- ↑ "Drug will save lives of accident victims, says study". BBC News Online. 2010. https://www.bbc.co.uk/news/10311371.
- ↑ "Tranexamic acid in trauma: how should we use it?". The Journal of Trauma and Acute Care Surgery 74 (6): 1575–1586. June 2013. doi:10.1097/TA.0b013e318292cc54. PMID 23694890.
- ↑ ((CRASH-3 trial collaborators)) (November 2019). "Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial". Lancet 394 (10210): 1713–1723. doi:10.1016/S0140-6736(19)32233-0. PMID 31623894.
- ↑ "Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial". Obstetrics and Gynecology 116 (4): 865–75. 2010. doi:10.1097/AOG.0b013e3181f20177. PMID 20859150.
- ↑ "Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review". Acta Obstetricia et Gynecologica Scandinavica 91 (5): 529–37. 2012. doi:10.1111/j.1600-0412.2012.01361.x. PMID 22229782.
- ↑ "Rates of medical or surgical treatment for women with heavy menstrual bleeding: the ECLIPSE trial 10-year observational follow-up study". Health Technology Assessment 27 (17): 1–50. October 2023. doi:10.3310/JHSW0174. PMID 37924269.
- ↑ "The coil and medicines are both effective long-term treatments for heavy periods". NIHR Evidence. 8 March 2024. doi:10.3310/nihrevidence_62335. https://evidence.nihr.ac.uk/alert/the-coil-and-medicines-are-both-effective-long-term-treatments-for-heavy-periods/. Retrieved 12 April 2024.
- ↑ "Postpartum Haemorrhage, Prevention and Management (Green-top Guideline No. 52)". https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg52/.
- ↑ 30.0 30.1 "Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion". The Cochrane Database of Systematic Reviews (John Wiley & Sons, Ltd) 2011 (3). 2011. doi:10.1002/14651858.cd001886.pub4. PMID 21412876.
- ↑ "Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis". BMJ 344. 2012. doi:10.1136/bmj.e3054. PMID 22611164.
- ↑ "Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss". The British Journal of Surgery 100 (10): 1271–9. 2013. doi:10.1002/bjs.9193. PMID 23839785. http://researchonline.lshtm.ac.uk/1037027/1/Systematic%20review%2C%20meta-analysis%20and%20meta-regression%20of%20the%20effect%20of%20tranexamic%20acid_GREEN%20AAM.pdf. Retrieved 8 August 2019.
- ↑ RCPCH. "Evidence Statement Major trauma and the use of tranexamic acid in children Nov 2012". http://www.rcpch.ac.uk/system/files/protected/page/121112_TXA%20evidence%20statement_final%20v2.pdf.
- ↑ "Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery". Anesthesiology 102 (4): 727–32. 2005. doi:10.1097/00000542-200504000-00006. PMID 15791100.
- ↑ "The effectiveness of tranexamic acid on operative and perioperative blood loss in long-segment spinal fusions: a consecutive series of 119 primary procedures". Journal of Neurosurgery. Spine 32 (5): 768–774. 2020. doi:10.3171/2019.11.SPINE191174. PMID 31978874.
- ↑ "Cyklokapron (tranexamic acid) Product Information". http://www.anzca.edu.au/communications/anzca-e-newsletter/e-news-advertisements/Product%20information%20-%20Pfizer.pdf.
- ↑ "Tranexamic acid in control of haemorrhage after dental extraction in haemophilia and Christmas disease". British Medical Journal 2 (5809): 311–3. 1972. doi:10.1136/bmj.2.5809.311. PMID 4553818.
- ↑ "Antifibrinolytic therapy for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing minor oral surgery or dental extractions". The Cochrane Database of Systematic Reviews 2019 (4). 2019. doi:10.1002/14651858.CD011385.pub3. PMID 31002742.
- ↑ "牙膏可添加氨甲环酸" (in zh). http://health.people.com.cn/n1/2018/1030/c14739-30370803.html.
- ↑ "LC Paper No. CB(3) 61/07-08 Proposed resolution under the Pharmacy and Poisons Ordinance". 7 November 2007. https://www.legco.gov.hk/yr07-08/english/hc/papers/hc1102cb3-61-e.pdf.
- ↑ "牙膏含"氨甲环酸"是处方药?看看牙膏里的秘密". 中国新闻周刊 [China News Weekly]. 31 October 2018. https://news.sina.com.cn/c/2018-10-31/doc-ifxeuwws9727254.shtml.
- ↑ "How to Choose Toothpaste". https://www.lion.co.jp/en/oral/point/02.htm. "if toothpaste has a label of "quasi drug", this indicates that the product contains active ingredients [...] Tranexamic acid"
- ↑ "Unauthorized". Health Canada. Government of Canada. 4 October 2019. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/71191a-eng.php.
- ↑ 44.0 44.1 "Antifibrinolytics (lysine analogues) for the prevention of bleeding in people with haematological disorders". The Cochrane Database of Systematic Reviews 2016 (3). 2016. doi:10.1002/14651858.CD009733.pub3. PMID 26978005.
- ↑ "Tranexamic acid". http://www.isbtweb.org/working-parties/clinical-transfusion/5-tranexamic-acid/.
- ↑ "How I treat target-specific oral anticoagulant-associated bleeding". Blood 123 (8): 1152–8. 2014. doi:10.1182/blood-2013-09-529784. PMID 24385535.
- ↑ "Topical application of tranexamic acid for the reduction of bleeding". The Cochrane Database of Systematic Reviews (7). 2013. doi:10.1002/14651858.CD010562.pub2. PMID 23881695. PMC 12053458. https://researchonline.lshtm.ac.uk/1105220/1/Topical%20application%20of%20tranexamic%20acid_GREEN%20VoR.pdf. Retrieved 30 November 2019.
- ↑ "Role of topical tranexamic acid in the management of idiopathic anterior epistaxis in adult patients in the emergency department". American Journal of Health-System Pharmacy 73 (21): 1755–1759. 2016. doi:10.2146/ajhp150829. PMID 27769971.
- ↑ "Haematological factors in the management of adult epistaxis: systematic review". The Journal of Laryngology and Otology 131 (12): 1093–1107. 2017. doi:10.1017/S0022215117002067. PMID 29280698.
- ↑ "Tranexamic Acid for the Treatment of Epistaxis". Academic Emergency Medicine 26 (11): 1292–1293. 2019. doi:10.1111/acem.13760. PMID 30933392.
- ↑ "Influence of pH on skin permeation of amino acids". The Journal of Pharmacy and Pharmacology 48 (7): 675–9. July 1996. doi:10.1111/j.2042-7158.1996.tb03949.x. PMID 8866327.
- ↑ "Applications of microneedling for various dermatologic indications with a special focus on pigmentary disorders: A comprehensive review study". Dermatologic Therapy 34 (6). November 2021. doi:10.1111/dth.15159. PMID 34657363.
- ↑ "Biodegradable derivatives of tranexamic acid as transdermal permeation enhancers". Journal of Controlled Release 104 (1): 41–49. May 2005. doi:10.1016/j.jconrel.2005.01.002. PMID 15866333.
- ↑ "The double prodrug concept and its applications". Advanced Drug Delivery Reviews 3 (1): 39–65. January 1989. doi:10.1016/0169-409x(89)90004-5.
- ↑ "Cutaneous metabolism in transdermal drug delivery". Current Drug Metabolism 10 (3): 227–35. March 2009. doi:10.2174/138920009787846350. PMID 19442085.
- ↑ "Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: A systematic review and meta-analysis". Thrombosis Research 179: 81–86. 2019. doi:10.1016/j.thromres.2019.05.003. PMID 31100632.
- ↑ 57.0 57.1 "Association of Intravenous Tranexamic Acid With Thromboembolic Events and Mortality: A Systematic Review, Meta-analysis, and Meta-regression". JAMA Surgery 156 (6): e210884. April 2021. doi:10.1001/jamasurg.2021.0884. PMID 33851983.
- ↑ "Tranexamic Acid use while Breastfeeding". 2014. https://www.drugs.com/breastfeeding/tranexamic-acid.html.
- ↑ 59.0 59.1 "Substans - Tranexamsyra". FASS.se. https://www.fass.se/LIF/substance?userType=0&substanceId=IDE4POCLU9M3PVERT1.
- ↑ "Drug Approval Package: Lysteda (tranexamic acid) NDA #022430". 6 May 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022430_lysteda_toc.cfm.
- ↑ "Xanodyne Announces FDA Approval of Lysteda (Tranexamic Acid) for Treatment of Women with Heavy Menstrual Bleeding". Drugs.com (Press release). Archived from the original on 31 July 2022. Retrieved 11 December 2020.
- ↑ "Tranexamic Acid to be available OtC". Community pharmacy news, analysis and CPD. 27 January 2011. http://www.chemistanddruggist.co.uk/news-content/-/article_display_list/4544044/4544040.
- ↑ "In defence of multiple pharmacies". Community pharmacy news, analysis and CPD. 10 February 2011. http://dotpharmacy.cmpi.biz/c/portal/layout?p_l_id=259751&CMPI_SHARED_articleId=4577833&CMPI_SHARED_ImageArticleId=4577833&CMPI_SHARED_CommentArticleId=4577833&CMPI_SHARED_ToolsArticleId=4577833&CMPI_SHARED_articleIdRelated=4577833.
- ↑ "Using antifibrinolytics to tackle neuroinflammation". Neural Regeneration Research 15 (12): 2203–2206. 2020. doi:10.4103/1673-5374.284979. PMID 32594031.
- ↑ "WHO | WHO updates recommendation on intravenous tranexamic acid for the treatment of postpartum haemorrhage". https://www.who.int/reproductivehealth/tranexamic-acid-pph-treatment/en/.
- ↑ "Does tranexamic acid stop haemoptysis?". Interactive Cardiovascular and Thoracic Surgery 17 (6): 991–4. 2013. doi:10.1093/icvts/ivt383. PMID 23966576.
- ↑ "Antifibrinolytic therapy to reduce haemoptysis from any cause". The Cochrane Database of Systematic Reviews 2016 (11). 2016. doi:10.1002/14651858.CD008711.pub3. PMID 27806184. PMC 6464927. http://www.cochrane.org/CD008711/TOBACCO_antifibrinolytic-therapy-reduce-haemoptysis. Retrieved 5 July 2018.
- ↑ Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. 2007. ISBN 978-0-443-06911-6. https://archive.org/details/rangdalespharmac0006dale.
- ↑ "Intranasal tranexamic acid treatment for severe epistaxis in hereditary hemorrhagic telangiectasia". Archives of Internal Medicine 161 (5): 767. 2001. doi:10.1001/archinte.161.5.767. PMID 11231712.
- ↑ "Melasma: systematic review of the systemic treatments". International Journal of Dermatology 56 (9): 902–908. 2017. doi:10.1111/ijd.13578. PMID 28239840.
- ↑ "Tranexamic acid in treatment of melasma: A comprehensive review of clinical studies". Dermatologic Therapy 30 (3). May 2017. doi:10.1111/dth.12465. PMID 28133910.
- ↑ "Quasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disorders". International Journal of Molecular Sciences 11 (6): 2566–2575. June 2010. doi:10.3390/ijms11062566. PMID 20640168.
- ↑ "Medical interventions for traumatic hyphema". The Cochrane Database of Systematic Reviews 2023 (3). March 2023. doi:10.1002/14651858.CD005431.pub5. PMID 36912744.
- ↑ "Tranexamic Acid in Patients Undergoing Liver Resection: The HeLiX Randomized Clinical Trial". JAMA 332 (13): 1080–1089. August 2024. doi:10.1001/jama.2024.11783. PMID 39158894.
 |
