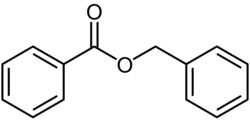
Chemistry:Benzyl benzoate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ascabin, Ascabiol, Ascarbin, Tenutex, others |
| Other names | phenylmethyl ester, benzoic ester |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H12O2 |
| Molar mass | 212.248 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.118 g/cm3 g/cm3 |
| Melting point | 18 °C (64 °F) |
| Boiling point | 323 °C (613 °F) |
| Solubility in water | insoluble mg/mL (20 °C) |
| |
| |
Benzyl benzoate is an organic compound which is used as a medication and insect repellent.[1] As a medication it is used to treat scabies and lice.[2] For scabies either permethrin or malathion is typically preferred.[3] It is applied to the skin as a lotion.[2] Typically two to three applications are needed.[2] It is also present in Balsam of Peru, Tolu balsam, and in a number of flowers.[4]
Side effects may include irritation of the skin.[2] It is not recommended in children.[3] It is also used in other animals; however, it is considered toxic to cats.[1] How it works is unclear.[5]
Benzyl benzoate was first studied medically in 1918.[1] It is on the World Health Organization's List of Essential Medicines.[6] Benzyl benzoate is sold under the brand name Scabanca among others and is available as a generic medication.[1][3] It is not available for medical use in the United States .[1]
Uses
Medical
Benzyl benzoate is an effective and inexpensive topical treatment for human scabies.[7] It has vasodilating and spasmolytic effects and is present in many asthma and whooping cough drugs.[8] It is also used as an excipient in some testosterone-replacement medications (like Nebido) for treating hypogonadism.[9]
Benzyl benzoate is used as a topical acaricide, scabicide, and pediculicide in veterinary hospitals.[10]
Non-medical
Benzyl benzoate is used as a repellent for chiggers, ticks, and mosquitoes.[10] It is also used as a dye carrier, solvent for cellulose derivatives, plasticizer, and fixative in the perfume industry.[11]
Side effects
Benzyl benzoate has low acute toxicity in laboratory animals. It is rapidly hydrolyzed to benzoic acid and benzyl alcohol. Benzyl alcohol is subsequently metabolized to benzoic acid. The conjugates of benzoic acid (hippuric acid and the glucuronide of benzoic acid) are rapidly eliminated in urine.[1] When given in large doses to laboratory animals, benzyl benzoate can cause hyperexcitation, loss of coordination, ataxia, convulsions, and respiratory paralysis.[10]
Benzyl benzoate can be a skin irritant when used as a topical scabicide.[7] Overdose can result in blistering and hives or a rash can occur as an allergic reaction.[12][13]
As an excipient in some testosterone-replacement injectable medications, benzyl benzoate has been reported as a cause of anaphylaxis in a case in Australia.[14] Bayer includes this report in information for health professionals and recommends that physicians "should be aware of the potential for serious allergic reactions" to preparations of this type.[9] In Australia, reports to ADRAC, which evaluates reports of adverse drug reactions for the Therapeutic Goods Administration, show several reports of allergic issues since the anaphylaxis case from 2011.
Chemistry
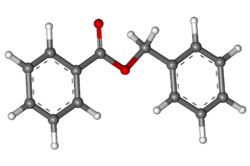
It is an organic compound with the formula C6H5CH2O2CC6H5. It is the ester of benzyl alcohol and benzoic acid. It forms either a viscous liquid or solid flakes and has a weak, sweet-balsamic odor. It occurs in a number of blossoms (e. g. tuberose, hyacinth) and is a component of Balsam of Peru and Tolu balsam.[11][4]
Production
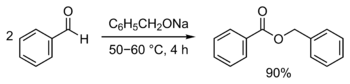
Benzyl benzoate is produced industrially by the reaction of sodium benzoate with benzyl chloride in the presence of a base, or by transesterification of methyl benzoate and benzyl alcohol.[8] It is a byproduct of benzoic acid synthesis by toluene oxidation.[11] It can also be synthesized by the Tishchenko reaction, using benzaldehyde with sodium benzyloxide (generated from sodium and benzyl alcohol) as catalyst:[15][16]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "22.4.2 Benzyl Benzoate". Handbook of Pesticide Toxicology, Volume 3: Classes of Pesticides. Academic Press. 1991. pp. 1505–1508. ISBN 0123341639. https://books.google.com/books?id=WiIlBQAAQBAJ&pg=PA1505.
- ↑ 2.0 2.1 2.2 2.3 WHO Model Formulary 2008. World Health Organization. 2009. p. 311. ISBN 9789241547659.
- ↑ 3.0 3.1 3.2 British National Formulary: BNF 69 (69 ed.). British Medical Association. 2015. p. 836. ISBN 9780857111562.
- ↑ 4.0 4.1 Ullmann's Encyclopedia of Industrial Chemistry. Wiley. 15 January 2003. doi:10.1002/14356007.a11_141. ISBN 3527306730.
- ↑ (in en) Georgis' Parasitology for Veterinarians. Elsevier Health Sciences. 2009. p. 262. ISBN 978-1416044123. https://books.google.com/books?id=g_tBWVBevM0C&pg=PA262.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 7.0 7.1 Tony Burns, ed. (2010), "Diseases Caused by Arthropods and Other Noxious Animals", Rook's Textbook of Dermatology, 2 (8th ed.), Wiley-Blackwell, p. 38.41
- ↑ 8.0 8.1 Ullmann's Encyclopedia of Industrial Chemistry. Wiley. 15 June 2000. doi:10.1002/14356007.a04_001. ISBN 3527306730.
- ↑ 9.0 9.1 "Nebido Monograph – Information for Health Care Professionls". Bayer. 2016. http://www.nebido.com/en/hcp/product-information/nebido-monograph/index.php.
- ↑ 10.0 10.1 10.2 Wexler, Philip, ed (2005). "Benzyl Benzoate". Encyclopedia of Toxicology. 1 (2nd ed.). Elsevier. pp. 264–265.
- ↑ 11.0 11.1 11.2 Ullmann's Encyclopedia of Industrial Chemistry. Wiley. 15 June 2000. doi:10.1002/14356007.a03_555. ISBN 3527306730.
- ↑ "Benzyl Benzoate (Topical route)". PubMed Health at the United States National Library of Medicine. 1 October 2016. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0009254/?report=details#side_effects.
- ↑ "Benzocaine-Benzyl Benzoate Topical: Side Effects". WebMD. 2016. http://www.webmd.com/drugs/2/drug-77581/benzocaine-benzyl-benzoate-topical/details/list-sideeffects.
- ↑ "Anaphylaxis triggered by benzyl benzoate in a preparation of depot testosterone undecanoate". Case Reports in Medicine 2012: 384054. 2012. doi:10.1155/2012/384054. 384054. PMID 22272209.
- ↑ Ullmann's Encyclopedia of Industrial Chemistry. Wiley. 15 October 2011. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3527306732.
- ↑ "Benzyl benzoate". Organic Syntheses 2: 5. 1922. doi:10.15227/orgsyn.002.0005. http://www.orgsyn.org/demo.aspx?prep=cv1p0104.; Collective Volume, 1, pp. 104
External links
- "Benzyl Benzoate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/benzyl%20benzoate.
 |