Chemistry:Borane carbonyl
| |

| |
| Names | |
|---|---|
| IUPAC name
Borane carbonyl
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| H 3BCO | |
| Molar mass | 41.84 g·mol−1 |
| Appearance | colorless gas |
| Density | 1.71 g/L[1] |
| Melting point | −137[1] °C (−215 °F; 136 K) |
| Boiling point | −64[1] °C (−83 °F; 209 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Borane carbonyl is the inorganic compound with the formula H
3BCO. This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared by combining borane-ether complexes and CO. The compound is mainly of theoretical and pedagogical interest.[2]
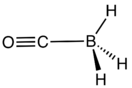
Structure and properties
The structure of the molecule of borane carbonyl is H
3B−
–C≡O+
. The B–C≡O linkage is linear. The coordination geometry around the boron atom is tetrahedral. The bond distances are 114.0 pm for the C≡O bond, 152.9 pm for the C–B bond, and 119.4 pm for the B–H bonds. The H–B–H bond angle is 113.7°. The C≡O vibrational band is at 2164.7 cm−1, around 22 cm−1 higher than that of free CO.[3]
Borane carbonyl has an enthalpy of vaporization of 19.7 kJ/mol (4750 cal/mol).[4] It has electronic state 1A1 and point group symmetry C3v.[5]
Synthesis and reactions
Borane carbonyl was discovered in 1937 by reacting diborane with excess carbon monoxide, with the equation:
- B
2H
6 + 2 CO ⇌ 2 BH
3CO.[4]
The reaction quickly reaches equilibrium at 100°C, but at room temperature, the reverse reaction is slow enough to isolate borane carbonyl. This reaction is performed at high pressures, typically with a maximum pressure observed of 1000 to 1600 psi (68.95 to 110.32 bar).[6] It can also be performed at atmospheric pressure, with ethers as a catalyst.[7][8]
A more recent synthesis of borane carbonyl involves slowly bubbling carbon monoxide through a 1 M H
3B–THF solution. The resulting gas stream can be condensed and subsequently bubbled through ethanolic potassium hydroxide to produce the boranocarbonate anion ([H
3BCO
2]2− or H
3B−
–CO−
2).[8]
References
- ↑ 1.0 1.1 1.2 "Borine carbonyl | 13205-44-2". https://www.chemicalbook.com/ChemicalProductProperty_EN_CB72358168.htm.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 165. ISBN 978-0-08-037941-8.
- ↑ Jacobsen, H.; Berke, H.; Doering, S.; Kehr, G.; Erker, G.; Froehlich, R.; Meyer, O. (1999). "Lewis Acid Properties of Tris(pentafluorophenyl)borane. Structure and Bonding in L-B(C6F5)3 Complexes". Organometallics 18: 1724–1735. doi:10.1021/OM981033E.
- ↑ 4.0 4.1 Burg, Anton B.; Schlesinger, H. I. (1937-05-01). "Hydrides of Boron. VII. Evidence of the Transitory Existence of Borine (BH 3 ): Borine Carbonyl and Borine Trimethylammine" (in en). Journal of the American Chemical Society 59 (5): 780–787. doi:10.1021/ja01284a002. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01284a002.
- ↑ NIST Chemistry WebBook. "NIST Chemistry WebBook". https://cccbdb.nist.gov/exp2x.asp?casno=13205442&charge=0.
- ↑ Carter, James C.; Parry, Robert W. (1965-06-01). "The Ammonia and Alkylamine Addition Compounds of Carbon Monoxide Borane". Journal of the American Chemical Society 87 (11): 2354–2358. doi:10.1021/ja01089a009. ISSN 0002-7863. http://dx.doi.org/10.1021/ja01089a009.
- ↑ Mayer, Erwin (1971-07-01). "Äther als Katalysatoren für die Reaktion von Diboran mit Lewis-Basen; vereinfachte Darstellung von Carbonylboran und Phosphinboran" (in de). Monatshefte für Chemie 102 (4): 940–945. doi:10.1007/BF00909917. ISSN 1434-4475. https://doi.org/10.1007/BF00909917.
- ↑ 8.0 8.1 Alberto, R.; Ortner, K.; Wheatley, N.; Schibli, R.; Schubiger, A. P. (2001). "Synthesis and Properties of Boranocarbonate: A Convenient in Situ CO Source for the Aqueous Preparation of [99mTc(OH2)3(CO)3]+". J. Am. Chem. Soc. 123 (13): 3135–3136. doi:10.1021/ja003932b. PMID 11457025.
 |

