Chemistry:Bromochlorofluoromethane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Bromo(chloro)fluoromethane | |
| Other names
Bromochlorofluoromethane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CHBrClF | |
| Molar mass | 147.37 g·mol−1 |
| Density | 1.953 g/cm3 |
| Melting point | −115 °C; −175 °F; 158 K |
| Boiling point | 36 °C; 97 °F; 309 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
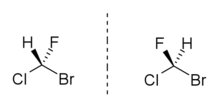
Bromochlorofluoromethane or fluorochlorobromomethane, is a chemical compound and trihalomethane derivative with the chemical formula CHBrClF. As one of the simplest possible stable chiral compounds, it is useful for fundamental research into this area of chemistry.[1] However, its relative instability to hydrolysis,[2] and lack of suitable functional groups, made separation of the enantiomers of bromochlorofluoromethane especially challenging,[3] and this was not accomplished until almost a century after it was first synthesised, in March 2005, though it has now been done by a variety of methods.[4][5][6][7] More recent research using bromochlorofluoromethane has focused on its potential use for experimental measurement of parity violation, a major unsolved problem in quantum physics.[8][9][10]
See also
- Bromochlorodifluoromethane, used in fire extinguishers
- Bromochlorofluoroiodomethane, a theoretical derivative with iodine replacing the hydrogen
References
- ↑ Berry, Kenneth L.; Sturtevant, Julian M. (1942). "Fluorochlorobromomethane". Journal of the American Chemical Society 64 (7): 1599–1600. doi:10.1021/ja01259a031.
- ↑ Thomas L. Gilchrist. Comprehensive Organic Functional Group Transformations. Volume 6. Synthesis: Carbon with Three or Four Attached Heteroatoms. p228. Pergamon / Elsevier, 1995. ISBN:0-08-042704-9
- ↑ Hargreaves, Michael K.; Modarai, Borzoo (1969). "An optically active haloform: (+)-bromochlorofluoromethane". Journal of the Chemical Society D: Chemical Communications 1: 16. doi:10.1039/C29690000016.
- ↑ Canceill, Josette; Lacombe, Liliane; Collet, Andre (1985). "Analytical optical resolution of bromochlorofluoromethane by enantioselective inclusion into a tailor-made cryptophane and determination of its maximum rotation". Journal of the American Chemical Society 107 (24): 6993–6996. doi:10.1021/ja00310a041.
- ↑ Doyle, Thomas R.; Vogl, Otto (1989). "Bromochlorofluoromethane and deuteriobromochlorofluoromethane of high optical purity". Journal of the American Chemical Society 111 (22): 8510–8511. doi:10.1021/ja00204a029.
- ↑ Grosenick, Heiko; Schurig, Volker; Costante, Jeanne; Collet, André (1995). "Gas chromatographic enantiomer separation of bromochlorofluoromethane". Tetrahedron: Asymmetry 6 (1): 87–88. doi:10.1016/0957-4166(94)00358-I.
- ↑ Pitzer, M.; Kunitski, M.; Johnson, A. S.; Jahnke, T.; Sann, H.; Sturm, F.; Schmidt, L. P. H.; Schmidt-Bocking, H. et al. (2013). "Direct Determination of Absolute Molecular Stereochemistry in Gas Phase by Coulomb Explosion Imaging". Science 341 (6150): 1096–1100. doi:10.1126/science.1240362. PMID 24009390. Bibcode: 2013Sci...341.1096P. https://tuprints.ulb.tu-darmstadt.de/24248/1/Science.pdf.
- ↑ Crassous, J.; Collet, A. (2000). "The bromochlorofluoromethane saga". Enantiomer 5 (5): 429–438. PMID 11143807.
- ↑ Crassous, Jeanne; Monier, Franck; Dutasta, Jean-Pierre; Ziskind, Michaël; Daussy, Christophe; Grain, Christophe; Chardonnet, Christian (2003). "Search for Resolution of Chiral Fluorohalogenomethanes and Parity-Violation Effects at the Molecular Level". ChemPhysChem 4 (6): 541–548. doi:10.1002/cphc.200200536. PMID 12836475.
- ↑ Darquié, Benoît; Stoeffler, Clara; Shelkovnikov, Alexander; Daussy, Christophe; Amy-Klein, Anne; Chardonnet, Christian; Zrig, Samia; Guy, Laure et al. (2010). "Progress toward the first observation of parity violation in chiral molecules by high-resolution laser spectroscopy". Chirality 22 (10): 870–884. doi:10.1002/chir.20911. PMID 20839292.
 |

