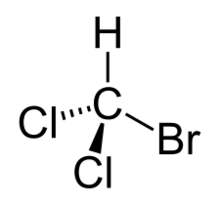
Chemistry:Bromodichloromethane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Bromo(dichloro)methane | |
| Other names
Bromodichloromethane
Dichlorobromomethane | |
| Identifiers | |
3D model (JSmol)
|
|
| 1697005 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 25941 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2810 3082 |
| |
| |
| Properties | |
| CHBrCl2 | |
| Molar mass | 163.8 g/mol |
| Appearance | Colorless liquid |
| Density | 1.980 g/cm3 |
| Melting point | −57 °C (−71 °F; 216 K) |
| Boiling point | 90 °C (194 °F; 363 K) |
| 4.5 g/L at 20 °C | |
| -66.3·10−6 cm3/mol | |
Refractive index (nD)
|
1.4964 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H315, H319, H335, H350 | |
| P201, P202, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P304+340, P305+351+338, P308+313, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bromodichloromethane is a trihalomethane with formula CHBrCl
2.
Bromodichloromethane has formerly been used as a flame retardant, and a solvent for fats and waxes and because of its high density for mineral separation. Now it is only used as a reagent or intermediate in organic chemistry.
Bromodichloromethane can also occur in municipally-treated drinking water as a by-product of the chlorine disinfection process.[1]
According to the Environmental Working Group, a non-profit organization that strives to educate consumers about potential chemical and environmental health risks, bromodichloromethane can increase the risk of cancer, harm to reproduction and child development, and may cause changes to fetal growth and development in when present in quantities higher than 0.06 parts per billion (ppb).[2] This data largely comes from studies reviewed or conducted by the California Office of Environmental Health Hazard Assessment. [3] No standards regulating the presence of bromodichloromethane in drinking water currently exist in the United States.[4]
Notes
- ↑ Agency for Toxic Substances & Disease Registry, Accessed 07/10/2012, https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsLanding.aspx?id=707&tid=127
- ↑ Group, Environmental Working. "EWG's Tap Water Database: Contaminants in Your Water" (in en). https://www.ewg.org/tapwater/contaminant.php?contamcode=2943?contamcode=2943.
- ↑ "Public Health Goals for Trihalomethanes in Drinking Water". https://oehha.ca.gov/media/downloads/water/chemicals/phg/thmsphg020720.pdf.
- ↑ Group, Environmental Working. "EWG's Tap Water Database: Disinfection Byproducts" (in en). https://www.ewg.org/tapwater/reviewed-disinfection-byproducts.php.
External links
- International Chemical Safety Card 0393
- Bromodichloromethane at The Carcinogenic Potency Database
- Toxicological Profile at ATSDR
 |

