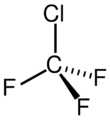
Chemistry:Chlorotrifluoromethane
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chloro(trifluoro)methane | |||
| Other names
Chlorotrifluoromethane
Monochlorotrifluoromethane Trifluorochloromethane Trifluoromethyl chloride Trifluoromonochlorocarbon Arcton 3 Freon 13 Genetron 13 R-13 CFC 13 UN 1022 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| CClF3 | |||
| Molar mass | 104.46 g/mol | ||
| Appearance | Colorless gas with sweet odor | ||
| Density | 1.526 g/cm3 | ||
| Melting point | −181 °C (−293.8 °F; 92.1 K) | ||
| Boiling point | −81.5 °C (−114.7 °F; 191.7 K) | ||
| 0.009% at 25 °C (77 °F) | |||
| Vapor pressure | 3.263 MPa at 21 °C (70 °F) | ||
| Thermal conductivity | 0.01217 W m−1 K−1 (300 K)[1] | ||
| Hazards | |||
| Main hazards | Ozone depletor and asphyxiant | ||
| Safety data sheet | ICSC 0420 | ||
| Flash point | Non-flammable | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive, nontoxic chlorofluorocarbon (CFC) and also a mixed halomethane. It is a man-made substance used primarily as a refrigerant. When released into the environment, CFC-13 has a high ozone depletion potential, and long atmospheric lifetime.[2] Only a few other greenhouse gases surpass CFC-13 in global warming potential (GWP).[3]:2 The IPCC AR5 reported that CFC-13's atmospheric lifetime was 640 years.[4]
Production
CFC-13—like all chlorofluorocarbon compounds—contains atoms of carbon (C), chlorine (Cl), and fluorine (F).[5][6]
It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride:
CCl4 + 3HF → CClF3 + 3HCl
This reaction can also produce trichlorofluoromethane (CCl3F), dichlorodifluoromethane (CCl2F2) and tetrafluoromethane (CF4).[7]
Montreal Protocol
Following the unanimous ratification of the 1987 Montreal Protocol—in response to concerns about the role of concentrations of chlorofluorocarbons (CFCs) in ozone layer-depletion in the stratosphere—a process was put into place to gradually phase out and replace CFC-13 and all the other CFCs.[8] Research in the 1980s said that these man-made CFC compound compounds had opened a hole in ozone layer in the upper atmosphere or stratosphere that protects life on earth from UV radiation.[5]
CFC-13's ozone depletion potential (ODP) is high— 1[9] (CCl3F = 1)—it is categorized as a Class I in the IPCC's list of ozone-depleting substances.[9] CFC-13's radiative efficiency is high which results in a high global warming potential (GWPs) of 13 900 GWP-100 yr that is "surpassed by very few other greenhouse gases."[3] It is categorized as a Class I in the list of ozone-depleting Substances.[9]:2
Increase in atmospheric abundance of CFC-13 in 2010s
Starting in the 2010s, despite a global ban on the production of CFCs, five of these ozone-damaging emissions were on the rise.[5]
The atmospheric abundance of CFC-13 rose from 3.0 parts per trillion (ppt) in year 2010 to 3.3 ppt in year 2020 based on analysis of air samples gathered from sites around the world.[10] Contrary to the Montreal Protocol, the atmospheric emissions of CFC-13 and four other chlorofluorocarbons (CFCs), increased between 2010 and 2020.[11]
As of 2023, the drivers behind the increase in CFC-13 and CFC-112a emissions were not certain.[11]
Physical properties
The IPCC AR5 reported that CFC-13's Atmospheric lifetime was 640 years.[12]
| Property | Value |
|---|---|
| Density (ρ) at -127.8 °C (liquid) | 1.603 g⋅cm−3 |
| Density (ρ) at boiling point (gas) | 6.94 kg⋅m−3 |
| Density (ρ) at 15 °C (gas) | 4.41 g⋅cm−3 |
| Triple point temperature (Tt) | |
| Critical temperature (Tc) | 28.8 °C (302 K) |
| Critical pressure (pc) | 3.86 MPa (38.6 bar) |
| Critical density (ρc) | 5.5 mol⋅L−1 |
| Latent heat of vaporization at boiling point | 149.85 kJ⋅kg−1 |
| Specific heat capacity at constant pressure (Cp) at -34.4 °C | 0.06 kJ⋅mol−1⋅K−1 |
| Specific heat capacity at constant volume (CV) at -34.4 °C | 0.051 kJ⋅mol−1⋅K−1 |
| Heat capacity ratio (к) at -34.4 °C | 1.168016 |
| Compressibility Factor (Z) at 15 °C | 0.9896 |
| Acentric factor (ω) | 0.17166 |
| Viscosity (η) at 0 °C (gas) | 13.3 mPa⋅s (0.0133 cP) |
| Viscosity (η) at 25 °C (gas) | 14.1 mPa⋅s (0.01440 cP) |
| Ozone depletion potential (ODP) | 1[9](CCl3F = 1) |
| Global warming potential (GWP) | 14,000[4] (CO2 = 1) |
| Atmospheric lifetime | 640 years[4] |
See also
References
- ↑ Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter - the TPRC data series. Volume 3. Thermal conductivity - nonmetallic liquids and gases. Data book. 1970.
- ↑ Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- ↑ 3.0 3.1 Vollmer, Martin; Young, Dickon; Trudinger, Cathy; Mühle, Jens; Henne, Stephan; Rigby, Matt; Park, Sunyoung; Li, Shihong et al. (October 10, 2017). "Atmospheric histories and emissions of chlorofluorocarbons CFC-13 (CClF3), CFC-114 (C2Cl2F4), and CFC-115 (C2ClF5)". Atmospheric Chemistry and Physics Discussions 2017 (39). doi:10.5194/acp-2017-935.
- ↑ 4.0 4.1 4.2 "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis. p. 731. https://www.ipcc.ch/report/ar5/wg1/.
- ↑ 5.0 5.1 5.2 Ashworth, James (April 3, 2023). "Mystery emissions of ozone-damaging gases are fuelling climate change". Natural History Museum. https://www.nhm.ac.uk/discover/news/2023/april/mystery-emissions-ozone-damaging-gases-fuelling-climate-change.html. Retrieved April 3, 2023.
- ↑ Elkins, James.W. (2013). "Halocarbons and other Atmospheric Trace Species". NOAA Global Monitoring Laboratory (Press release). Retrieved April 3, 2023 – via US Department of Commerce and NOAA.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 304. ISBN 978-0-08-037941-8.
- ↑ Allen, Kate (April 3, 2023). "Remember ozone-destroying CFCs? They're on the rise again. And the source is a mystery". The Star. https://www.thestar.com/news/canada/2023/04/03/remember-ozone-destroying-cfcs-theyre-on-the-rise-again-and-the-source-is-a-mystery.html.
- ↑ 9.0 9.1 9.2 9.3 "Class I Ozone-depleting Substances". Science - Ozone Layer Protection. US EPA. 2007. http://www.epa.gov/Ozone/science/ods/classone.html.
- ↑ "AGAGE Data and Figures". Massachusetts Institute of Technology. https://agage.mit.edu/data/agage-data. Retrieved 2021-02-11.
- ↑ 11.0 11.1 Western, Luke M.; Vollmer, Martin K.; Krummel, Paul B.; Adcock, Karina E.; Fraser, Paul J.; Harth, Christina M.; Langenfelds, Ray L.; Montzka, Stephen A. et al. (April 3, 2023). "Global increase of ozone-depleting chlorofluorocarbons from 2010 to 2020". Nature Geoscience 16 (4): 309–313. doi:10.1038/s41561-023-01147-w. ISSN 1752-0908. Bibcode: 2023NatGe..16..309W. https://www.nature.com/articles/s41561-023-01147-w. Retrieved April 3, 2023.
- ↑ Forster, Piers; Ramaswamy, Venkatachalam; Artaxo, Paulo; Berntsen, Terje; Betts, Richard; Fahey, David W; Haywood, James; Lean, Judith et al.. "Changes in Atmospheric Constituents and in Radiative Forcing". International Panel of Climate Change (IPCC). AR4 Climate Change 2007: The Physical Science Basis.
External links
- MSDS at mathesontrigas.com
- International Chemical Safety Card 0420
- Entry at Air Liquide gas encyclopaedia
- The crystal structure of chlorotrifluoromethane, CF3Cl; neutron powder diffraction and constrained refinement[yes|permanent dead link|dead link}}]
- Termochemical data table
 |