Chemistry:Calcium fumarate
From HandWiki
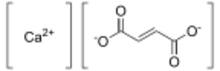
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | calcium+fumarate |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CaC4H2O4 | |
| Molar mass | 154.134 g mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319 | |
| P264, P280, P305+351+338, P337+313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcium fumarate is a compound with formula Ca(C2H2(COO)2) or (OOC-CH=CH-COO)Ca. It is a calcium salt of fumaric acid, and has been used to enrich foods to boost calcium absorption.[1]
References
- ↑ Weaver, Connie M; Martin, Berdine R; Costa, Neuza M B; Saleeb, Fouad Z; Huth, Peter J (2002). "Absorption of Calcium Fumarate Salts Is Equivalent to Other Calcium Salts When Measured in the Rat Model". Journal of Agricultural and Food Chemistry 50 (17): 4974–4975. doi:10.1021/jf0200422. PMID 12166992.
- ↑ "FOOD ADDITIVES : "E" NUMBERS". http://www.ufrgs.br/imunovet/molecular_immunology/foodadditives.html.
 |
