Chemistry:Compounds of palladium(III)
In chemistry, compounds of palladium(III) feature the noble metal palladium in the unusual +3 oxidation state (in most of its compounds, palladium has the oxidation state II). Compounds of Pd(III) occur in mononuclear and dinuclear forms. Palladium(III) is most often invoked, not observed in mechanistic organometallic chemistry.[1][2][2]
Mononuclear compounds
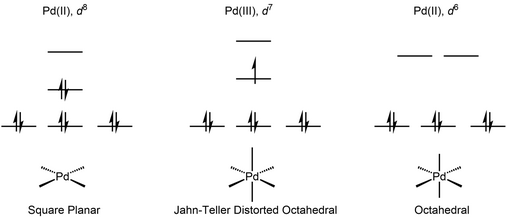
Pd(III) has a d7 electronic configuration, which leads to a Jahn-Teller distorted octahedral geometry. The geometry could also be viewed as being intermediate between square-planar and octahedral. These complexes are low-spin and paramagnetic.
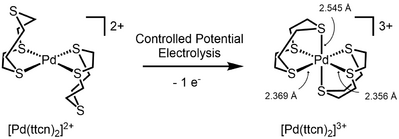
The first Pd(III) complex characterized by X-ray crystallography was reported in 1987.[3] It was obtained by oxidation of the 1,4,7-trithiacyclononane (ttcn) complex [Pd(ttcn)2]3+. X-Ray crystallography revealed the expected Jahn-Teller distorted octahedral geometry, in spite of the highly symmetric structure of the ligand.
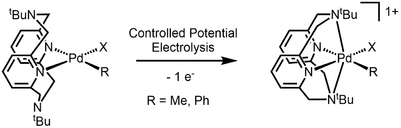
The first organometallic Pd(III) complex characterized by X-Ray crystallography was reported in 2010.[4] Organopalladium complexes supported with a macrocyclic tetradentate ligand undergo single-electron oxidation to give Pd(III) species that is stabilized by the axially-positioned amine. The authors propose that while the axial nitrogen stabilize a distorted octahedral geometry, the t-Bu group and the rigidity of the macrocyclic structure inhibits the oxidation to a more conventional octahedral Pd(IV).
Dinuclear compounds
Structure
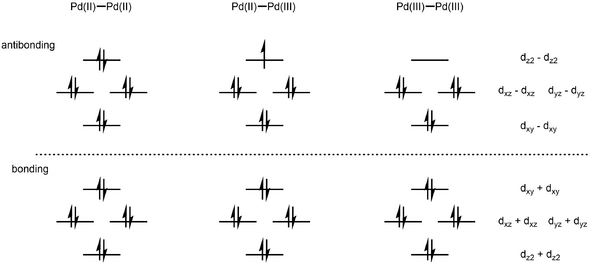
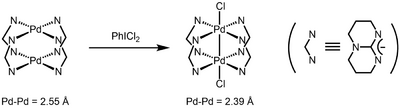
Pairs of Pd(III) centers can couple, giving rise to a Pd–Pd bond order of 1.[5] A two-electron oxidation of two Pd(II) species can generate a diamagnetic, Pd(III)-Pd(III) dimer with a bond order of 1.
The first example of a dipalladium(III) complex was obtained by oxidation of dinuclear Pd(II) complex of triazabicyclodecene.[6]
The first organometallic dinuclear Pd(III) complexes were reported in 2006 by Cotton and coworkers as well.[7] These complexes catalyze the diborylation of terminal olefins.[8] Due to the facile reduction of these complexes to Pd(II) species by diborane, the authors proposed that the dinuclear Pd(III) complexes serve as precatalysts for active Pd(II) catalysts.
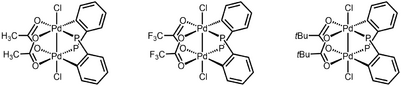
Reactivity
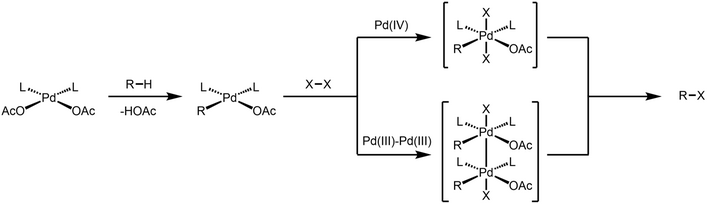
The reactivity of dinuclear Pd(III) species as active catalytic intermediate is mostly discussed in the context of C-H activation. While it was proposed that Pd-catalyzed oxidative C-H functionalization reactions involve a Pd(IV) intermediate, Ritter and coworkers first postulated that these oxidative reactions could involve a dinuclear Pd(III) intermediate instead of Pd(IV).[9]
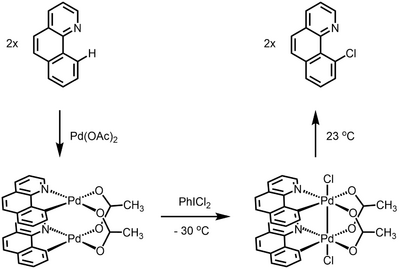
Dinuclear Pd species are involved in Pd-catalyzed C-H chlorination.[10] Through X-ray crystallography, Ritter unambiguously showed that dinuclear Pd(III) complex is formed when the palladacycle is treated with two-electron oxidant, and such dinuclear complex undergoes C-Cl reductive elimination under ambient temperature. Both experimental and computational data was consistent with a concerted 1,1-reductive elimination mechanism for the C-Cl forming step.[11][12] The authors show that such bimetallic participation of redox event lowers the activation barrier for reductive elimination step by ~30 kcal/mol compared to a monometallic pathway.
Acetoxylation of 2-phenylpyridine was also demonstrated to involve a dinuclear Pd(III) intermediate.[13]
References
- ↑ Mirica, Liviu M.; Khusnutdinova, Julia R. (2013-01-15). "Structure and electronic properties of Pd(III) complexes". Coordination Chemistry Reviews 257 (2): 299–314. doi:10.1016/j.ccr.2012.04.030.
- ↑ 2.0 2.1 Powers, David C.; Ritter, Tobias (2011). Canty, Allan J.. ed (in en). Higher Oxidation State Organopalladium and Platinum Chemistry. Topics in Organometallic Chemistry. 503. Springer Berlin Heidelberg. pp. 129–156. doi:10.1007/978-3-642-17429-2_6. ISBN 9783642174285.
- ↑ Blake, Alexander J.; Holder, Alan J.; Hyde, Timothy I.; Schröder, Martin (January 1987). "Stabilisation of mononuclear palladium(III). The single crystal X-ray structure of the [Pd(L)2]3+cation (L = 1,4,7-trithiacyclononane)" (in en). J. Chem. Soc., Chem. Commun. 0 (13): 987–988. doi:10.1039/c39870000987. ISSN 0022-4936.
- ↑ Khusnutdinova, Julia R.; Rath, Nigam P.; Mirica, Liviu M. (2010). "Stable Mononuclear Organometallic Pd(III) Complexes and Their C−C Bond Formation Reactivity". Journal of the American Chemical Society 132 (21): 7303–7305. doi:10.1021/ja103001g. ISSN 0002-7863. PMID 20462195.
- ↑ Albert Cotton, F.; Murillo, Carlos A.; Walton, Richard A. (2005) (in en). Multiple Bonds Between Metal Atoms - Springer. doi:10.1007/b136230. ISBN 978-0-387-25084-7.
- ↑ Cotton, F. Albert; Gu, Jiande; Murillo, Carlos A.; Timmons, Daren J. (1998). "The First Dinuclear Complex of Palladium(III)". Journal of the American Chemical Society 120 (50): 13280–13281. doi:10.1021/ja9832313. ISSN 0002-7863.
- ↑ Cotton, F. Albert; Koshevoy, Igor O.; Lahuerta, Pascual; Murillo, Carlos A.; Sanaú, Mercedes; Ubeda, M. Angeles; Zhao, Qinliang (2006). "High Yield Syntheses of Stable, Singly Bonded Pd26+ Compounds". Journal of the American Chemical Society 128 (42): 13674–13675. doi:10.1021/ja0656595. ISSN 0002-7863. PMID 17044680.
- ↑ Penno, Dirk; Lillo, Vanesa; Koshevoy, Igor O.; Sanaú, Mercedes; Ubeda, M. Angeles; Lahuerta, Pascual; Fernández, Elena (2008). "Multifaceted Palladium Catalysts Towards the Tandem Diboration–Arylation Reactions of Alkenes" (in en). Chemistry – A European Journal 14 (34): 10648–10655. doi:10.1002/chem.200800931. ISSN 1521-3765. PMID 18932177.
- ↑ Powers, David C.; Ritter, Tobias (2012). "Bimetallic Redox Synergy in Oxidative Palladium Catalysis". Accounts of Chemical Research 45 (6): 840–850. doi:10.1021/ar2001974. ISSN 0001-4842. PMID 22029861. http://nrs.harvard.edu/urn-3:HUL.InstRepos:10861159.
- ↑ Powers, David C.; Ritter, Tobias (July 2009). "Bimetallic Pd(III) complexes in palladium-catalysed carbon–heteroatom bond formation" (in en). Nature Chemistry 1 (4): 302–309. doi:10.1038/nchem.246. ISSN 1755-4330. Bibcode: 2009NatCh...1..302P.
- ↑ Powers, David C.; Benitez, Diego; Tkatchouk, Ekaterina; Goddard, William A.; Ritter, Tobias (2010). "Bimetallic Reductive Elimination from Dinuclear Pd(III) Complexes". Journal of the American Chemical Society 132 (40): 14092–14103. doi:10.1021/ja1036644. ISSN 0002-7863. PMID 20858006.
- ↑ Powers, David C.; Xiao, Daphne Y.; Geibel, Matthias A. L.; Ritter, Tobias (2010). "On the Mechanism of Palladium-Catalyzed Aromatic C−H Oxidation". Journal of the American Chemical Society 132 (41): 14530–14536. doi:10.1021/ja1054274. ISSN 0002-7863. PMID 20873835.
- ↑ Powers, David C.; Geibel, Matthias A. L.; Klein, Johannes E. M. N.; Ritter, Tobias (2009). "Bimetallic Palladium Catalysis: Direct Observation of Pd(III)−Pd(III) Intermediates". Journal of the American Chemical Society 131 (47): 17050–17051. doi:10.1021/ja906935c. ISSN 0002-7863. PMID 19899740. http://nrs.harvard.edu/urn-3:HUL.InstRepos:8156526.
P