Chemistry:Endothall
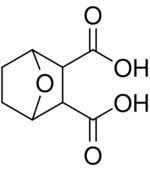
| |
| Names | |
|---|---|
| IUPAC name
7-Oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid
| |
| Other names
Endothal; 3,6-Endoxohexahydrophthalic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H10O5 | |
| Molar mass | 186.163 g·mol−1 |
| Density | 1.431 g/cm3 (20 °C)[2] |
| Melting point | 144 °C (291 °F; 417 K)[2] |
| 100 g/L (20 °C)[2] | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
38 mg/kg (oral, rat)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Endothall (3,6-endoxohexahydrophthalic acid) is used as an herbicide for terrestrial and aquatic plants. It is used as an aquatic herbicide for submerged aquatic plants and algae in lakes, ponds and irrigation canals.[3] It is used, as a desiccant on potatoes, hops, cotton, clover and alfalfa. It is used as a biocide to control mollusks and algae in cooling towers.
Endothall is a selective contact herbicide that has been used to manage submerged aquatic vegetation for over 50 years. The herbicide damages the cells of susceptible plants at the point of contact but does not affect areas untouched by the herbicide, like roots or tubers (underground storage structures).[4]
The chemical formula for endothall is C8H10O5. Its Chemical Abstracts Service (CAS) name is 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid. It is an organic acid but is used as the dipotassium salt or the mono-N, N-dimethylalkylamine salt.[5] It is considered safe in drinking water by the EPA up to a maximum contaminant level of 0.1 mg/L (100 ppb). Some people who drink water contaminated above this level for many years experience stomach or intestine problems.[6]
Endothall is chemically related to cantharidin.[7] Both compounds are protein phosphatase 2A inhibitors.[8]
See also
References
- ↑ Menninger, Holly. "Error: no
|title=specified when using {{Cite web}}". Cornell Cooperative Extension. https://ccetompkins.org/environment/aquatic-invasives/hydrilla/management-options/herbicides/endothall/endothall-faq. - ↑ 2.0 2.1 2.2 2.3 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ "Herbicides against Hydrilla". Tompkins County NY website.
- ↑ Cornell Cooperative Extension, Tompkins County, Endothall FAQ,
- ↑ "Reregistration Eligibility Decision for Endothall". EPA. Archived from the original on 16 October 2014. https://web.archive.org/web/20141016104429/http://www.epa.gov/oppsrrd1/REDs/endothall_red.pdf. Retrieved 16 July 2012.
- ↑ "Basic Information about Endothall in Drinking Water". EPA. http://water.epa.gov/drink/contaminants/basicinformation/endothall.cfm. Retrieved 16 July 2012.
- ↑ Li, YM; Casida, JE (15 December 1992). "Cantharidin-binding protein: identification as protein phosphatase 2A.". Proceedings of the National Academy of Sciences of the United States of America 89 (24): 11867–70. doi:10.1073/pnas.89.24.11867. PMID 1334551. Bibcode: 1992PNAS...8911867L.
- ↑ Liu, Ji-Yuan; Chen, Xi-En; Zhang, Ya-Lin (20 July 2015). "Insights into the key interactions between human protein phosphatase 5 and cantharidin using molecular dynamics and site-directed mutagenesis bioassays". Scientific Reports 5 (1): 12359. doi:10.1038/srep12359. PMID 26190207. Bibcode: 2015NatSR...512359L.
- P. Chris Wilson and Jun Wu. "SL369/SS570: Aquatic Toxicology Notes: Endothall". ufl.edu. Archived from the original on 2014-09-09. https://web.archive.org/web/20140909095020/http://edis.ifas.ufl.edu/ss570.
- http://www.apms.org/japm/vol08a/v8p50.pdf
- http://dnr.wi.gov/lakes/plants/factsheets/EndothallFactsheet.pdf
 |

