Chemistry:Linuron
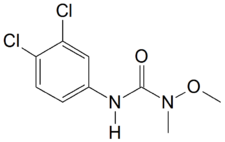 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C9H10Cl2N2O2 |
| Molar mass | 249.09 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Linuron (3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea) is a phenylurea herbicide[1] that is used to control the growth of grass and weeds for the purpose of supporting the growth of crops like soybeans.[2][3]
Pharmacology
Mechanism of action
Linuron acts via inhibition of photosystem II, which is necessary for photosynthetic electron transport in plants.[2][3]
Effects in animals
Linuron has been found to produce reproductive toxicity in animals by acting as an androgen receptor (AR) antagonist, and for this reason, is considered to be an endocrine disruptor.[2][4] Consequently, in January 2017, the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF) of the European Commission DG "Health and food safety" decided to not renew its regulatory approval.[5] Sales are expected to cease by June 2017.[5]
See also
- Diuron
- Monolinuron
References
- ↑ "Linuron and monolinuron". Residue Reviews. Reviews of Environmental Contamination and Toxicology 77: 1–364. 1981. doi:10.1007/978-1-4612-5874-2_1. ISBN 978-1-4612-5876-6. PMID 7017855.
- ↑ 2.0 2.1 2.2 Understanding Toxicology. Jones & Bartlett Learning. 30 August 2016. pp. 705–. ISBN 978-1-284-12761-4. https://books.google.com/books?id=OQ0hDQAAQBAJ&pg=PA705.
- ↑ 3.0 3.1 Metabolic Pathways of Agrochemicals. Royal Society of Chemistry. 1998. pp. 744–. ISBN 978-0-85404-494-8. https://books.google.com/books?id=uC2-Ocob_MMC&pg=PA744.
- ↑ "Peer review of the pesticide risk assessment of the active substance linuron". EFSA Journal 14 (7). July 2016. doi:10.2903/j.efsa.2016.4518.
- ↑ 5.0 5.1 Curtis, Marianne. "Linuron fails to gain renewed approval". Briefing Media Ltd. https://www.fginsight.com/news/linuron-fails-to-gain-renewed-approval-17923.
 |
