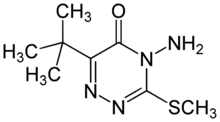
Chemistry:Metribuzin
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Amino-5-tert-butyl-2-(methylsulfanyl)-1,2,4-triazin-5(4H)-one | |
| Other names
4-Amino-6-(1,1-dimethylethyl)-3-(methylthio)-1,2,4-triazin-5(4H)-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H14N4OS | |
| Molar mass | 214.29 g·mol−1 |
| Appearance | Colorless, crystalline solid[1] |
| Density | 1.31 g/cm3 |
| Melting point | 125 °C (257 °F; 398 K) |
| 0.1% (20 °C)[1] | |
| Vapor pressure | 0.0000004 mmHg (20 °C)[1] |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
5 mg/m3[1] |
IDLH (Immediate danger)
|
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Metribuzin (4-amino-6-tert-butyl-3-(methylthio)-1,2,4-triazin-5(4H)-one) is a herbicide used both pre- and post-emergence in crops including soy bean, potatoes, tomatoes and sugar cane.
It acts by inhibiting photosynthesis by disrupting photosystem II.[2] It is widely used in agriculture and has been found to contaminate groundwater.[3]
Metribuzin is produced by reacting one mole of 4-amino-6-tert-butyl-3-mercapto-(1,2,4)triazin-5(4H)one and half a mole of dimethyl sulfonate which react at 57°C in presence of sulfuric acid media about 7 hours and transfer methyl (CH3) from triazine to metribuzin and product formed 1 mole of metribuzin and half mole of sulfuric acid and later neutralized with soda ash and then purified.[citation needed]
MP=125°C, BP=132°C, and cause dust explosion if enough amount of energy absorbed by it.[citation needed]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 NIOSH Pocket Guide to Chemical Hazards. "#0430". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0430.html.
- ↑ Terence Robert Roberts; David Herd Hutson (17 July 1998). Metabolic Pathways of Agrochemicals: Herbicides and plant growth regulators. Royal Society of Chemistry. pp. 662–. ISBN 978-0-85404-494-8. https://books.google.com/books?id=uC2-Ocob_MMC&pg=PA662. Retrieved 25 May 2012.
- ↑ Undabeytia, T. S.; Recio, E.; Maqueda, C.; Morillo, E.; Gómez-Pantoja, E.; Sánchez-Verdejo, T. (2011). "Reduced metribuzin pollution with phosphatidylcholine-clay formulations". Pest Management Science 67 (3): 271–278. doi:10.1002/ps.2060. PMID 21308953.
External links
- Metribuzin in the Pesticide Properties DataBase (PPDB)
 |
