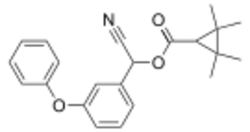
Chemistry:Fenpropathrin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C22H23NO3 |
| Molar mass | 349.430 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fenpropathrin, or fenopropathrin, is a widely used pyrethroid insecticide in agriculture and household.[1][2][3][4] Fenpropathrin is an ingestion and contact synthetic pyrethroid. Its mode of action is similar to other natural (pyrethrum) and synthetic pyrethroids where in they interfere with the kinetics of voltage gated sodium channels causing paralysis and death of the pest.[5] Fenpropathrin was the first of the light-stable synthetic pyrethroids to be synthesized in 1971, but it was not commercialized until 1980.[6] Like other pyrethroids with an α-cyano group, fenpropathrin also belongs to the termed type II pyrethroids (e.g. cyfluthrin, cyhalothrin, cypermethrin, deltamethrin and esfenvalerate).[7] Type II pyrethroids are a more potent toxicant than type I in depolarizing insect nerves.[8] Application rates of fenpropathrin in agriculture according to US environmental protection agency (EPA) varies by crop but is not to exceed 0.4 lb ai/acre.
Toxicity
A person developed Parkinson's disease after six months of daily exposure to fenpropathrin, and animal tests subsequently revealed that the compound is a dopaminergic neurotoxin.[4] The patient had a history of eating fenpropathrin-poisoned fish for 6 months.[4] The follow-up Dopaminergic degeneration study was conducted using mice treated with fenpropathrin at 15mg/kg/day for 60 days.[4] It has thus been implicated as an environmental risk factor for Parkinson's disease[4] similar to organochlorines, organophosphates and pyrethroids especially at higher doses.[9][10][11][5] An acute reference dose for chronic dietary exposure for fenpropathrin is set at 0.025/mg/kg/day by US EPA. Fenpropathrin is toxic to bees if they come in contact with them directly similar to other insecticides.[12] Toxicity dissipates with time when deposited on foliage and is <24 hours.
Environmental Fate
Fenpropathrin degrades from soil by two main mechanisms, biodegradation and photochemical degradation of surface deposits.[13] The time of degradation depends on the characteristics of the soils.[7] The half-life of disappearance for fenpropathrin in soils was 11 to 17 days under aerobic conditions and approx >1 yr under anaerobic conditions. The half-life of fenpropathrin on the surface of a sterilized sandy loam was in the range of 3 to 4 days following irradiation with natural sunlight.
Trade Names
Danitol, Meothrin, Tame.
See also
- 6-Hydroxydopamine
- MPTP
- Norsalsolinol
- Rotenone
References
- ↑ Metabolic Maps of Pesticides. Elsevier Science. 2 December 2012. pp. 185–. ISBN 978-0-323-15753-7. https://books.google.com/books?id=Cnv3hTkZyo0C&pg=PA185.
- ↑ Neurotoxicology. CRC Press. 15 July 1992. pp. 462–. ISBN 978-1-4398-0542-8. https://books.google.com/books?id=CBZ8An2koJkC&pg=PA462.
- ↑ Pollinator Protection: A Bee & Pesticide Handbook. Wicwas Press. 1990. ISBN 978-1-878075-00-0. https://books.google.com/books?id=UutJAAAAYAAJ.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Fenpropathrin, a Widely Used Pesticide, Causes Dopaminergic Degeneration". Molecular Neurobiology 53 (2): 995–1008. March 2016. doi:10.1007/s12035-014-9057-2. PMID 25575680.
- ↑ 5.0 5.1 "Pesticide Residues: Pyrethroids" (in en). Encyclopedia of Food Safety. Elsevier. 2014. pp. 31–34. doi:10.1016/B978-0-12-378612-8.00239-0. ISBN 978-0-12-378613-5.
- ↑ "Selectivity of action between pyrethroids and combined DDT-pyrethroid insecticides on Na+ influx into mammalian neuroblastoma". Experientia 41 (4): 520–522. 1985. doi:10.1007/bf01966180. ISSN 0014-4754.
- ↑ 7.0 7.1 Pesticide residues in food, 1993 : evaluations, 1993. Part II, Toxicology. World Health Organization, International Program on Chemical Safety. Geneva, Switzerland: World Health Organization. 1994. ISBN 92-4-166509-2. OCLC 31097583. https://www.worldcat.org/oclc/31097583.
- ↑ "The importance of nerve terminal depolarization in pyrethroid poisoning of insects" (in en). Pesticide Biochemistry and Physiology 20 (2): 169–182. 1983. doi:10.1016/0048-3575(83)90021-4.
- ↑ "Environmental Exposures and Parkinson's Disease". International Journal of Environmental Research and Public Health 13 (9): 881. September 2016. doi:10.3390/ijerph13090881. PMID 27598189.
- ↑ "Occupational exposure to pesticides and Parkinson's disease: a systematic review and meta-analysis of cohort studies". Environment International 46: 30–43. October 2012. doi:10.1016/j.envint.2012.05.004. PMID 22698719.
- ↑ "Neurological Deficits After Long-term Pyrethroid Exposure". Environmental Health Insights 11: 1178630217700628. 2017-01-01. doi:10.1177/1178630217700628. PMID 28469448.
- ↑ "Evaluation of potential miticide toxicity to Varroa destructor and honey bees, Apis mellifera, under laboratory conditions". Scientific Reports 10 (1): 21529. December 2020. doi:10.1038/s41598-020-78561-2. PMID 33299084. Bibcode: 2020NatSR..1021529B.
- ↑ "864. Fenpropathrin (Pesticide residues in food: 1993 evaluations Part II Toxicology)". http://www.inchem.org/documents/jmpr/jmpmono/v93pr10.htm.
