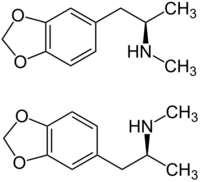
Chemistry:MDMA
3,4-Methylenedioxymethamphetamine (MDMA), commonly known as ecstasy (tablet form), and molly (crystal form),[1][2] is an entactogen with stimulant and minor psychedelic properties.[3][4][5]
MDMA was first synthesized in 1912 by Merck chemist Anton Köllisch.[6] It was used to enhance psychotherapy beginning in the 1970s and became popular as a street drug in the 1980s.[7][8] MDMA is commonly associated with dance parties, raves, and electronic dance music.[9] Tablets sold as ecstasy may be mixed with other substances such as ephedrine, amphetamine, and methamphetamine.[7] In 2016, about 21 million people between the ages of 15 and 64 used ecstasy (0.3% of the world population).[10] In the United States, as of 2017, about 7% of people have used MDMA at some point in their lives and 0.9% have used it in the last year.[11] The lethal risk from one dose of MDMA is estimated to be from 1 death in 20,000 instances to 1 death in 50,000 instances.[12]
The purported pharmacological effects that may be prosocial include altered sensations, increased energy, empathy, and pleasure.[5][7] When taken by mouth, effects begin in 30 to 45 minutes and last three to six hours.[13][8] Short-term adverse effects include grinding of the teeth, blurred vision, sweating, and a rapid heartbeat,[7] and extended use can also lead to addiction, memory problems, paranoia, and difficulty sleeping. Deaths have been reported due to increased body temperature and dehydration. MDMA acts primarily by increasing the release of the neurotransmitters serotonin, dopamine, and norepinephrine in parts of the brain.[7][8] It belongs to the substituted amphetamine classes of drugs.[14][15] MDMA is structurally similar to mescaline (a psychedelic), methamphetamine (a stimulant), as well as endogenous monoamine neurotransmitters such as serotonin, norepinephrine, and dopamine.[16]
MDMA has limited approved medical uses in a small number of countries,[17] but is illegal in most jurisdictions.[18] MDMA-assisted psychotherapy is a promising and generally safe treatment for post-traumatic stress disorder when administered in controlled therapeutic settings.[19][20] In the United States, the Food and Drug Administration (FDA) has given MDMA breakthrough therapy status (though there no current clinical indications in the US).[21] Canada has allowed limited distribution of MDMA upon application to and approval by Health Canada.[22] In Australia, it may be prescribed in the treatment of PTSD by specifically authorised psychiatrists.[23]
Uses
Recreational
MDMA is often considered the drug of choice within the rave culture and is also used at clubs, festivals, and house parties.[24] In the rave environment, the sensory effects of music and lighting are often highly synergistic with the drug. The psychedelic amphetamine quality of MDMA offers multiple appealing aspects to users in the rave setting. Some users enjoy the feeling of mass communion from the inhibition-reducing effects of the drug, while others use it as party fuel because of the drug's stimulatory effects.[25] MDMA is used less often than other stimulants, typically less than once per week.[26]
MDMA is sometimes taken in conjunction with other psychoactive drugs such as LSD,[27] psilocybin mushrooms, 2C-B, and ketamine. The combination with LSD is called "candy-flipping".[27] The combination with 2C-B is called "nexus flipping". For this combination, most people take the MDMA first, wait until the peak is over, and then take the 2C-B.[28]
MDMA is often co-administered with alcohol, methamphetamine, and prescription drugs such as SSRIs with which MDMA has several drug-drug interactions.[29][30][31] Three life-threatening reports of MDMA co-administration with ritonavir have been reported;[32] with ritonavir having severe and dangerous drug-drug interactions with a wide range of both psychoactive, anti-psychotic, and non-psychoactive drugs.[33]
Medical
As of 2023, MDMA therapies have only been approved for research purposes, with no widely accepted medical indications,[14][34][35] although this varies by jurisdiction. Before it was widely banned, it saw limited use in psychotherapy.[36][14][37] In 2017 the United States Food and Drug Administration (FDA) granted breakthrough therapy designation for MDMA-assisted psychotherapy for post-traumatic stress disorder (PTSD).[38][39]
Some researchers have proposed that psychedelics in general may act as active "super placebos" used for therapeutic purposes.[40][41]
Others
Small doses of MDMA are used by some religious practitioners as an entheogen to enhance prayer or meditation.[42] MDMA has been used as an adjunct to New Age spiritual practices.[43]
Forms

MDMA has become widely known as ecstasy (shortened "E", "X", or "XTC"), usually referring to its tablet form, although this term may also include the presence of possible adulterants or diluents. The UK term "mandy" and the US term "molly" colloquially refer to MDMA in a crystalline powder form that is thought to be free of adulterants.[44][45][46] MDMA is also sold in the form of the hydrochloride salt, either as loose crystals or in gelcaps.[47][48] MDMA tablets can sometimes be found in a shaped form that may depict characters from popular culture. These are sometimes collectively referred to as "fun tablets".[49][50]
Partly due to the global supply shortage of sassafras oil—a problem largely assuaged by use of improved or alternative modern methods of synthesis—the purity of substances sold as molly have been found to vary widely. Some of these substances contain methylone, ethylone, MDPV, mephedrone, or any other of the group of compounds commonly known as bath salts, in addition to, or in place of, MDMA.[45][46][47][48] Powdered MDMA ranges from pure MDMA to crushed tablets with 30–40% purity.[14] MDMA tablets typically have low purity due to bulking agents that are added to dilute the drug and increase profits (notably lactose) and binding agents.[14] Tablets sold as ecstasy sometimes contain 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxyethylamphetamine (MDEA), other amphetamine derivatives, caffeine, opiates, or painkillers.[36] Some tablets contain little or no MDMA.[36][14][51] The proportion of seized ecstasy tablets with MDMA-like impurities has varied annually and by country.[14] The average content of MDMA in a preparation is 70 to 120 mg with the purity having increased since the 1990s.[36]
MDMA is usually consumed by mouth. It is also sometimes snorted.[7]
Effects
In general, MDMA users report feeling the onset of subjective effects within 30 to 60 minutes of oral consumption and reaching peak effect at 75 to 120 minutes, which then plateaus for about 3.5 hours.[52] The desired short-term psychoactive effects of MDMA have been reported to include:
- Euphoria – a sense of general well-being and happiness[5][53]
- Increased self-confidence, sociability, and perception of facilitated communication[36][5][53]
- Entactogenic effects—increased empathy or feelings of closeness with others[5][53] and oneself[36]
- Dilated pupils[36]
- Relaxation and reduced anxiety[36]
- Increased emotionality[36]
- A sense of inner peace[53]
- Mild hallucination[53]
- Enhanced sensation, perception, or sexuality[36][5][53]
- Altered sense of time[8]
The experience elicited by MDMA depends on the dose, setting, and user.[36] The variability of the induced altered state is lower compared to other psychedelics. For example, MDMA used at parties is associated with high motor activity, reduced sense of identity, and poor awareness of surroundings. Use of MDMA individually or in small groups in a quiet environment and when concentrating, is associated with increased lucidity, concentration, sensitivity to aesthetic aspects of the environment, enhanced awareness of emotions, and improved capability of communication.[24][54] In psychotherapeutic settings, MDMA effects have been characterized by infantile ideas, mood lability, and memories and moods connected with childhood experiences.[54][55]
MDMA has been described as an "empathogenic" drug because of its empathy-producing effects.[56][57] Results of several studies show the effects of increased empathy with others.[56] When testing MDMA for medium and high doses, it showed increased hedonic and arousal continuum.[58][59] The effect of MDMA increasing sociability is consistent, while its effects on empathy have been more mixed.[60]
Side effects
Short-term
Acute adverse effects are usually the result of high or multiple doses, although single dose toxicity can occur in susceptible individuals.[5] The most serious short-term physical health risks of MDMA are hyperthermia and dehydration.[53][61] Cases of life-threatening or fatal hyponatremia (excessively low sodium concentration in the blood) have developed in MDMA users attempting to prevent dehydration by consuming excessive amounts of water without replenishing electrolytes.[53][61][12]
The immediate adverse effects of MDMA use can include:
- Bruxism (grinding and clenching of the teeth)[36][24][5]
- Dehydration[24][53][61]
- Diarrhea[53]
- Erectile dysfunction[36][62]
- Hyperthermia[36][24][61]
- Increased wakefulness or insomnia[36][53]
- Increased perspiration and sweating[53][61]
- Increased heart rate and blood pressure[36][24][61]
- Increased psychomotor activity[36]
- Loss of appetite[36][51]
- Nausea and vomiting[5]
- Visual and auditory hallucinations (rarely)[36]
Other adverse effects that may occur or persist for up to a week following cessation of moderate MDMA use include:[51][5]
- Physiological
- Psychological
Long-term
As of 2015[update], the long-term effects of MDMA on human brain structure and function have not been fully determined.[65] However, there is consistent evidence of structural and functional deficits in MDMA users with high lifetime exposure.[65] These structural or functional changes appear to be dose dependent and may be less prominent in MDMA users with only a moderate (typically <50 doses used and <100 tablets consumed) lifetime exposure. Nonetheless, moderate MDMA use may still be neurotoxic and what constitutes moderate use is not clearly established.[66]
Furthermore, it is not clear yet whether "typical" recreational users of MDMA (1 to 2 pills of 75 to 125 mg MDMA or analogue every 1 to 4 weeks) will develop neurotoxic brain lesions.[67] Long-term exposure to MDMA in humans has been shown to produce marked neurodegeneration in striatal, hippocampal, prefrontal, and occipital serotonergic axon terminals.[65][68] Neurotoxic damage to serotonergic axon terminals has been shown to persist for more than two years.[68] Elevations in brain temperature from MDMA use are positively correlated with MDMA-induced neurotoxicity.[24][65][66] However, most studies on MDMA and serotonergic neurotoxicity in humans focus more on heavy users who consume as much as seven times or more the amount that most users report taking. The evidence for the presence of serotonergic neurotoxicity in casual users who take lower doses less frequently is not conclusive.[69]
However, adverse neuroplastic changes to brain microvasculature and white matter have been observed to occur in humans using low doses of MDMA.[24][65] Reduced gray matter density in certain brain structures has also been noted in human MDMA users.[24][65] Global reductions in gray matter volume, thinning of the parietal and orbitofrontal cortices, and decreased hippocampal activity have been observed in long term users.[36] The effects established so far for recreational use of ecstasy lie in the range of moderate to severe effects for serotonin transporter reduction.[70]
Impairments in multiple aspects of cognition, including attention, learning, memory, visual processing, and sleep, have been found in regular MDMA users.[36][5][71][65] The magnitude of these impairments is correlated with lifetime MDMA usage[5][71][65] and are partially reversible with abstinence.[36] Several forms of memory are impaired by chronic ecstasy use;[5][71] however, the effects for memory impairments in ecstasy users are generally small overall.[72][73] MDMA use is also associated with increased impulsivity and depression.[36]
Serotonin depletion following MDMA use can cause depression in subsequent days. In some cases, depressive symptoms persist for longer periods.[36] Some studies indicate repeated recreational use of ecstasy is associated with depression and anxiety, even after quitting the drug.[74] Depression is one of the main reasons for cessation of use.[36]
At high doses, MDMA induces a neuroimmune response that, through several mechanisms, increases the permeability of the blood–brain barrier, thereby making the brain more susceptible to environmental toxins and pathogens.[75][76] In addition, MDMA has immunosuppressive effects in the peripheral nervous system and pro-inflammatory effects in the central nervous system.[77]
MDMA may increase the risk of cardiac valvulopathy in heavy or long-term users due to activation of serotonin 5-HT2B receptors.[78][79] MDMA induces cardiac epigenetic changes in DNA methylation, particularly hypermethylation changes.[80]
Reinforcement disorders
Approximately 60% of MDMA users experience withdrawal symptoms when they stop taking MDMA.[51] Some of these symptoms include fatigue, loss of appetite, depression, and trouble concentrating.[51] Tolerance to some of the desired and adverse effects of MDMA is expected to occur with consistent MDMA use.[51] A 2007 delphic analysis of a panel of experts in pharmacology, psychiatry, law, policing and others estimated MDMA to have a psychological dependence and physical dependence potential roughly three-fourths to four-fifths that of cannabis.[81]
MDMA has been shown to induce ΔFosB in the nucleus accumbens.[82] Because MDMA releases dopamine in the striatum, the mechanisms by which it induces ΔFosB in the nucleus accumbens are analogous to other dopaminergic psychostimulants.[82][83] Therefore, chronic use of MDMA at high doses can result in altered brain structure and drug addiction that occur as a consequence of ΔFosB overexpression in the nucleus accumbens.[83] MDMA is less addictive than other stimulants such as methamphetamine and cocaine.[84][85] Compared with amphetamine, MDMA and its metabolite MDA are less reinforcing.[86]
One study found approximately 15% of chronic MDMA users met the DSM-IV diagnostic criteria for substance dependence.[87] However, there is little evidence for a specific diagnosable MDMA dependence syndrome because MDMA is typically used relatively infrequently.[26]
There are currently no medications to treat MDMA addiction.[88]
During pregnancy
MDMA is a moderately teratogenic drug (i.e., it is toxic to the fetus).[89][90] In utero exposure to MDMA is associated with a neuro- and cardiotoxicity[90] and impaired motor functioning. Motor delays may be temporary during infancy or long-term. The severity of these developmental delays increases with heavier MDMA use.[71][91] MDMA has been shown to promote the survival of fetal dopaminergic neurons in culture.[92]
Overdose
MDMA overdose symptoms vary widely due to the involvement of multiple organ systems. Some of the more overt overdose symptoms are listed in the table below. The number of instances of fatal MDMA intoxication is low relative to its usage rates. In most fatalities, MDMA was not the only drug involved. Acute toxicity is mainly caused by serotonin syndrome and sympathomimetic effects.[87] Sympathomimetic side effects can be managed with carvedilol.[93][94] MDMA's toxicity in overdose may be exacerbated by caffeine, with which it is frequently cut in order to increase volume.[95] A scheme for management of acute MDMA toxicity has been published focusing on treatment of hyperthermia, hyponatraemia, serotonin syndrome, and multiple organ failure.[96]
| System | Minor or moderate overdose[97] | Severe overdose[97] |
|---|---|---|
| Cardiovascular |
| |
| Central nervous system |
| |
| Musculoskeletal |
| |
| Respiratory | ||
| Urinary | ||
| Other |
Interactions
A number of drug interactions can occur between MDMA and other drugs, including serotonergic drugs.[51][101] MDMA also interacts with drugs which inhibit CYP450 enzymes, like ritonavir (Norvir), particularly CYP2D6 inhibitors.[51] Life-threatening reactions and death have occurred in people who took MDMA while on ritonavir.[102] Bupropion, a strong CYP2D6 inhibitor, has been found to increase MDMA exposure with administration of MDMA.[103][104] Concurrent use of MDMA with certain other serotonergic drugs can result in a life-threatening condition called serotonin syndrome.[36][51] Severe overdose resulting in death has also been reported in people who took MDMA in combination with certain monoamine oxidase inhibitors (MAOIs),[36][51] such as phenelzine (Nardil), tranylcypromine (Parnate), or moclobemide (Aurorix, Manerix).[105] Serotonin reuptake inhibitors (SRIs) such as citalopram (Celexa), duloxetine (Cymbalta), fluoxetine (Prozac), and paroxetine (Paxil) have been shown to block most of the subjective effects of MDMA.[106] Norepinephrine reuptake inhibitors (NRIs) such as reboxetine (Edronax) have been found to reduce emotional excitation and feelings of stimulation with MDMA but do not appear to influence its entactogenic or mood-elevating effects.[106]
MDMA induces the release of monoamine neurotransmitters and thereby acts as an indirectly acting sympathomimetic and produces a variety of cardiostimulant effects.[103] It dose-dependently increases heart rate, blood pressure, and cardiac output.[103][107] SRIs like citalopram and paroxetine, as well as the serotonin 5-HT2A receptor antagonist ketanserin, have been found to partially block the increases in heart rate and blood pressure with MDMA.[103][108] It is notable in this regard that serotonergic psychedelics such as psilocybin, which act as serotonin 5-HT2A receptor agonists, likewise have sympathomimetic effects.[109][110][111] The NRI reboxetine and the serotonin–norepinephrine reuptake inhibitor (SNRI) duloxetine block MDMA-induced increases in heart rate and blood pressure.[103] Conversely, bupropion, a norepinephrine–dopamine reuptake inhibitor (NDRI) with only weak dopaminergic activity,[112][113] reduced MDMA-induced heart rate and circulating norepinephrine increases but did not affect MDMA-induced blood pressure increases.[103][104] On the other hand, the robust NDRI methylphenidate, which has sympathomimetic effects of its own, has been found to augment the cardiovascular effects and increases in circulating norepinephrine and epinephrine levels induced by MDMA.[103][114]
The non-selective beta blocker pindolol blocked MDMA-induced increases in heart rate but not blood pressure.[103][93][115] The α2-adrenergic receptor agonist clonidine did not affect the cardiovascular effects of MDMA, though it reduced blood pressure.[103][93][116] The α1-adrenergic receptor antagonists doxazosin and prazosin blocked or reduced MDMA-induced blood pressure increases but augmented MDMA-induced heart rate and cardiac output increases.[103][93][117][107] The dual α1- and β-adrenergic receptor blocker carvedilol reduced MDMA-induced heart rate and blood pressure increases.[103][93][94] In contrast to the cases of serotonergic and noradrenergic agents, the dopamine D2 receptor antagonist haloperidol did not affect the cardiovascular responses to MDMA.[103][118] Due to the theoretical risk of "unopposed α-stimulation" and possible consequences like coronary vasospasm, it has been suggested that dual α1- and β-adrenergic receptor antagonists like carvedilol and labetalol, rather than selective beta blockers, should be used in the management of stimulant-induced sympathomimetic toxicity, for instance in the context of overdose.[93][119]
Pharmacology
Pharmacodynamics
| Target | Affinity (Ki, nM) |
|---|---|
| SERT | 0.73–13,300 (Ki) 380–2,500 (IC50) 50–72 (EC50) (rat) |
| NET | 27,000–30,500 (Ki) 360–405 (IC50) 54–110 (EC50) (rat) |
| DAT | 6,500–>10,000 (Ki) 1,440–21,000 (IC50) 51–278 (EC50) (rat) |
| 5-HT1A | 6,300–12,200 (Ki) 36,000 nM (EC50) 64% (Emax) |
| 5-HT1B | >10,000 |
| 5-HT1D | >10,000 |
| 5-HT1E | >10,000 |
| 5-HT1F | ND |
| 5-HT2A | 4,600–>10,000 (Ki) 6,100–12,484 (EC50) 40–55% (Emax) |
| 5-HT2B | 500–2,000 (Ki) 2,000–>20,000 (EC50) 32% (Emax) |
| 5-HT2C | 4,400–>13,000 (Ki) 831–9,100 (EC50) 92% (Emax) |
| 5-HT3 | >10,000 |
| 5-HT4 | ND |
| 5-HT5A | >10,000 |
| 5-HT6 | >10,000 |
| 5-HT7 | >10,000 |
| α1A | 6,900–>10,000 |
| α1B | >10,000 |
| α1D | ND |
| α2A | 2,532–15,000 |
| α2B | 1,785 |
| α2C | 1,123–1,346 |
| β1, β2 | >10,000 |
| D1 | >13,600 |
| D2 | 25,200 |
| D3 | >17,700 |
| D4 | >10,000 |
| D5 | >10,000 |
| H1 | 2,138–>14,400 |
| H2 | >10,000 |
| H3, H4 | ND |
| M1 | >10,000 |
| M2 | >10,000 |
| M3 | 1,850–>10,000 |
| M4 | 8,250–>10,000 |
| M5 | 6,340–>10,000 |
| nACh | >10,000 |
| TAAR1 | 250–370 (Ki) (rat) 1,000–1,700 (EC50) (rat) 56% (Emax) (rat) 2,400–3,100 (Ki) (mouse) 4,000 (EC50) (mouse) 71% (Emax) (mouse) 35,000 (EC50) (human) 26% (Emax) (human) |
| I1 | 220 |
| σ1, σ2 | ND |
| Notes: The smaller the value, the more avidly the drug binds to the site. Proteins are human unless otherwise specified. Refs:[120][121][3][122][123][124] [125][126][127][128][129][130] | |
MDMA is an entactogen or empathogen, as well as a stimulant, euphoriant, and weak psychedelic.[3][131] It is a substrate of the monoamine transporters (MATs) and acts as a monoamine releasing agent (MRA).[3][132][133][134] The drug is specifically a well-balanced serotonin–norepinephrine–dopamine releasing agent (SNDRA).[3][132][133][134] To a lesser extent, MDMA also acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI).[3][132][133] MDMA enters monoaminergic neurons via the MATs and then, via poorly understood mechanisms, reverses the direction of these transporters to produce efflux of the monoamine neurotransmitters rather than the usual reuptake.[3][135][136][137] Induction of monoamine efflux by amphetamines in general may involve intracellular Na+ and Ca2+ elevation and PKC and CaMKIIα activation.[135][136][137] MDMA also acts on the vesicular monoamine transporter 2 (VMAT2) on synaptic vesicles to increase the cytosolic concentrations of the monoamine neurotransmitters available for efflux.[3][132] By inducing release and reuptake inhibition of serotonin, norepinephrine, and dopamine, MDMA increases levels of these neurotransmitters in the brain and periphery.[3][132]
In addition to its actions as an SNDRA, MDMA directly but more modestly interacts with a number of monoamine and other receptors.[3][120][121][122][138] It is a low-potency partial agonist of the serotonin 5-HT2 receptors, including of the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors.[3][139][140][141][138] The drug also interacts with α2-adrenergic receptors, with the sigma σ1 and σ2 receptors, and with the imidazoline I1 receptor.[3][120][121][122] Along with the preceding receptor interactions, MDMA is a potent partial agonist of the rodent trace amine-associated receptor 1 (TAAR1).[128][129] Conversely however, due to species differences, it is far weaker in terms of potency as an agonist of the human TAAR1.[3][128][129][142] Moreover, MDMA appears to act as a weak partial agonist of the human TAAR1 rather than as an efficacious agonist.[128][129] In relation to the preceding findings, MDMA has been said to be essentially inactive as a human TAAR1 agonist.[3] TAAR1 activation is thought to auto-inhibit and constrain the effects of amphetamines that possess TAAR1 agonism, for instance MDMA in rodents.[132][143][144][123][145]
Elevation of serotonin, norepinephrine, and dopamine levels by MDMA is believed to mediate most of the drug's effects, including its entactogenic, stimulant, euphoriant, hyperthermic, and sympathomimetic effects.[3][132][146][60] The entactogenic effects of MDMA, including increased sociability, empathy, feelings of closeness, and reduced aggression, are thought to be mainly due to induction of serotonin release.[60][106][147] The exact serotonin receptors responsible for MDMA's entactogenic effects are unclear, but may include the serotonin 5-HT1A receptor,[148] 5-HT1B receptor,[149] and 5-HT2A receptor,[150] as well as 5-HT1A receptor-mediated oxytocin release and consequent activation of the oxytocin receptor.[3][60][151][152][131] Induction of dopamine release is thought to be importantly involved in the stimulant and euphoriant effects of MDMA,[3][139][153] while induction of norepinephrine release and serotonin 5-HT2A receptor stimulation are believed to mediate its sympathomimetic effects.[103][132] Activation of serotonin 5-HT1B and 5-HT2A receptors is also thought to be involved in the stimulant and euphoriant effects of MDMA, while serotonin 5-HT2C receptor activation is thought to constrain these effects and limit MDMA's reinforcing potential.[154][155][156][157][158][159] Serotonin 5-HT2B receptor signaling appears to be required for MDMA-induced serotonin release and effects.[160][161][162][163][164] MDMA has been associated with a unique subjective "magic" or euphoria that few or no other known entactogens are said to fully reproduce.[165][166] The mechanisms underlying this property of MDMA are unknown, but it has been theorized to be due to a specific mixture and balance of pharmacological activities, including combined serotonin, norepinephrine, and dopamine release and direct serotonin receptor agonism.[167][165][166][168]
MDMA is often said to have mild or weak psychedelic effects.[169][106][147][170] These effects are said to be dose-dependent, such that greater hallucinogenic effects are produced at higher doses.[169][171] The mild hallucinogenic effects of MDMA include perceptual changes like intensification of visual, auditory, and tactile perception (e.g., brightened colors), a state of dissociation with feelings of depersonalization and derealization (e.g., "oceanic boundlessness"), and thinking disturbances.[169][172][106][170][108][173][171] Conversely, overt hallucinations do not occur, MDMA's hallucinogenic effects are described as "non-problematic" for users, and are said to be much less than those of 3,4-methylenedioxyamphetamine (MDA) or especially those of fully effective serotonergic psychedelics like psilocybin.[172][108][147] The hallucinogenic effects of MDMA have been theorized to be mediated by serotonin 5-HT2A receptor activation analogously to the case of classical psychedelics.[169][108][171][174][172][175] Accordingly, the serotonin 5-HT2A receptor antagonist ketanserin has been reported to reduce MDMA-induced perceptual changes in humans.[169][106][108][171] Conversely however, it failed to affect MDMA-induced feelings of dissociation and oceanic boundlessness.[169][106][171] In contrast, the serotonin reuptake inhibitor citalopram, which blocks MDMA-induced serotonin release, diminished all of the psychoactive and hallucinogenic effects of MDMA.[169][106][173][108] It has been noted that N-methylation of psychedelic phenethylamines, as in the structural difference between MDA and MDMA, has invariably greatly reduced or abolished their psychedelic activity.[176][177] Whereas MDA and psychedelics like psilocybin induce the head-twitch response in rodents, a behavioral proxy of psychedelic effects, findings on MDMA and the head-twitch response are mixed and conflicting.[178][179][106] In addition, whereas MDA fully substitutes for psychedelics like LSD and DOM in rodent drug discrimination tests, MDMA does not do so, nor do psychedelics generally fully substitute for MDMA.[180][106][181][182]
Long-term repeated activation of serotonin 5-HT2B receptors by MDMA is thought to result in increased risk of organ complications such as valvular heart disease (VHD) and primary pulmonary hypertension (PPH).[183][184][109][185][167][186] MDMA has been associated with serotonergic neurotoxicity.[187][147][188] This may be due to formation of toxic MDMA metabolites and/or induction of simultaneous serotonin and dopamine release, with consequent uptake of dopamine into serotonergic neurons and breakdown into toxic species.[187][147][188] Serotonin 5-HT2 receptor agonists or serotonergic psychedelics may potentiate the neurotoxicity of MDMA.[189][190][191][192]
MDMA is a racemic mixture of two enantiomers, (S)-MDMA and (R)-MDMA.[139][175] (S)-MDMA is much more potent as an SNDRA in vitro and in producing MDMA-like subjective effects in humans than (R)-MDMA.[139][134][175][193] By contrast, (R)-MDMA acts as a lower-potency serotonin–norepinephrine releasing agent (SNRA) with weak or negligible effects on dopamine.[139][134][194] Relatedly, (R)-MDMA shows weak or negligible stimulant-like and rewarding effects in animals.[139][195] Both (S)-MDMA and (R)-MDMA produce entactogen-type effects in animals and humans.[139][175] In addition, both (S)-MDMA and (R)-MDMA are weak agonists of the serotonin 5-HT2 receptors.[139][153][175][140][141] (R)-MDMA is more potent and efficacious as a serotonin 5-HT2A and 5-HT2B receptor agonist than (S)-MDMA, whereas (S)-MDMA is somewhat more potent as an agonist of the serotonin 5-HT2C receptor.[139][153][175] Due to it being a more potent serotonin 5-HT2A receptor agonist than (S)-MDMA, (R)-MDMA has been hypothesized to have greater psychedelic effects than (S)-MDMA or racemic MDMA.[196][175] However, this proved not to be the case in a direct clinical comparison of (R)-MDMA, (S)-MDMA, and racemic MDMA, with equivalent hallucinogen-like effects instead found between the three interventions.[196][175]
MDMA produces MDA as a minor active metabolite.[97] Peak levels of MDA are about 5 to 10% of those of MDMA and total exposure to MDA is almost 10% of that of MDMA with oral MDMA administration.[97][184] As a result, MDA may contribute to some extent to the effects of MDMA.[97][174] MDA is an entactogen, stimulant, and weak psychedelic similarly to MDMA.[147] Like MDMA, it acts as a potent and well-balanced SNDRA and as a weak serotonin 5-HT2 receptor agonist.[134][140][141] However, MDA shows much more potent and efficacious serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptor agonism than MDMA.[153][174][141][140] Accordingly, MDA produces greater psychedelic effects than MDMA in humans[147] and might particularly contribute to the mild psychedelic-like effects of MDMA.[174] On the other hand, MDA may also be importantly involved in toxicity of MDMA, such as cardiac valvulopathy.[197][184][140]
The duration of action of MDMA (3–6 hours) is much shorter than its elimination half-life (8–9 hours) would imply.[198] In relation to this, MDMA's duration and the offset of its effects appear to be determined more by rapid acute tolerance rather than by circulating drug concentrations.[29] Similar findings have been made for amphetamine and methamphetamine.[199][200][201][202] One mechanism by which tolerance to MDMA may occur is internalization of the serotonin transporter (SERT).[203][204][205][206][207] Although MDMA and serotonin are not significant TAAR1 agonists in humans, TAAR1 activation by MDMA may result in SERT internalization, for instance in rodents in whom MDMA is a potent TAAR1 agonist.[206][207][208][128] It is thought that brain serotonin levels are depleted after MDMA administration but that levels typically return to normal within 24 to 48 hours.[36]
| Compound | Serotonin | Norepinephrine | Dopamine |
|---|---|---|---|
| Amphetamine | ND | ND | ND |
| (S)-Amphetamine (d) | 698–1,765 | 6.6–7.2 | 5.8–24.8 |
| (R)-Amphetamine (l) | ND | 9.5 | 27.7 |
| Methamphetamine | ND | ND | ND |
| (S)-Methamphetamine (d) | 736–1,292 | 12.3–13.8 | 8.5–24.5 |
| (R)-Methamphetamine (l) | 4,640 | 28.5 | 416 |
| MDA | 160 | 108 | 190 |
| MDMA | 49.6–72 | 54.1–110 | 51.2–278 |
| (S)-MDMA (d) | 74 | 136 | 142 |
| (R)-MDMA (l) | 340 | 560 | 3,700 |
| MDEA | 47 | 2,608 | 622 |
| MBDB | 540 | 3,300 | >100,000 |
| MDAI | 114 | 117 | 1,334 |
| Notes: The smaller the value, the more strongly the drug releases the neurotransmitter. The assays were done in rat brain synaptosomes and human potencies may be different. See also Monoamine releasing agent § Activity profiles for a larger table with more compounds. Refs:[134][140][209][210][211][212][213][214][3] | |||
| Compound | 5-HT2A | 5-HT2B | 5-HT2C | |||
|---|---|---|---|---|---|---|
| EC50 (nM) | Emax | EC50 (nM) | Emax | EC50 (nM) | Emax | |
| Serotonin | 53 | 92% | 1.0 | 100% | 22 | 91% |
| MDA | 1,700 | 57% | 190 | 80% | ND | ND |
| (S)-MDA (d) | 18,200 | 89% | 100 | 81% | 7,400 | 73% |
| (R)-MDA (l) | 5,600 | 95% | 150 | 76% | 7,400 | 76% |
| MDMA | 6,100 | 55% | 2,000–>20,000 | 32% | ND | ND |
| (S)-MDMA (d) | 10,300 | 9% | 6,000 | 38% | 2,600 | 53% |
| (R)-MDMA (l) | 3,100 | 21% | 900 | 27% | 5,400 | 27% |
| Notes: The smaller the Kact or EC50 value, the more strongly the compound produces the effect. Refs:[141][140][215] | ||||||
Pharmacokinetics
Absorption
The MDMA concentration in the bloodstream starts to rise after about 30 minutes,[216] and reaches its maximal concentration between 1.5 and 3 hours after oral administration.[217] It is then slowly metabolized and excreted, with levels of MDMA and its metabolites decreasing to half their peak concentration over the next several hours.[218] The duration of action of MDMA is about 3 to 6 hours.[147]
Distribution
The plasma protein binding of MDMA is unknown.[219]
Metabolism

Metabolites of MDMA that have been identified in humans include 3,4-methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxymethamphetamine (HMMA), 4-hydroxy-3-methoxyamphetamine (HMA), 3,4-dihydroxyamphetamine (DHA) (also called alpha-methyldopamine (α-Me-DA)), 3,4-methylenedioxyphenylacetone (MDP2P), and 3,4-methylenedioxy-N-hydroxyamphetamine (MDOH). The contributions of these metabolites to the psychoactive and toxic effects of MDMA are an area of active research. 80% of MDMA is metabolised in the liver, and about 20% is excreted unchanged in the urine.[24]
MDMA is known to be metabolized by two main metabolic pathways: (1) O-demethylenation followed by catechol-O-methyltransferase (COMT)-catalyzed methylation or glucuronide/sulfate conjugation; and (2) N-dealkylation, deamination, and oxidation to the corresponding benzoic acid derivatives conjugated with glycine.[97] The metabolism may be primarily by cytochrome P450 (CYP450) enzymes CYP2D6 and CYP3A4 and COMT. Complex, nonlinear pharmacokinetics arise via autoinhibition of CYP2D6 and CYP2D8, resulting in zeroth order kinetics at higher doses. It is thought that this can result in sustained and higher concentrations of MDMA if the user takes consecutive doses of the drug.[220][non-primary source needed]
Elimination
MDMA and metabolites are primarily excreted as conjugates, such as sulfates and glucuronides.[221] MDMA is a chiral compound and has been almost exclusively administered as a racemate. However, the two enantiomers have been shown to exhibit different kinetics. The disposition of MDMA may also be stereoselective, with the S-enantiomer having a shorter elimination half-life and greater excretion than the R-enantiomer. Evidence suggests[222] that the area under the blood plasma concentration versus time curve (AUC) was two to four times higher for the (R)-enantiomer than the (S)-enantiomer after a 40 mg oral dose in human volunteers. Likewise, the plasma half-life of (R)-MDMA was significantly longer than that of the (S)-enantiomer (5.8 ± 2.2 hours vs 3.6 ± 0.9 hours).[51] However, because MDMA excretion and metabolism have nonlinear kinetics,[223] the half-lives would be higher at more typical doses (100 mg is sometimes considered a typical dose).[217]
Chemistry
| |
Reactors used to synthesize MDMA on an industrial scale in a clandestine chemical factory in Cikande, Indonesia |
MDMA is in the substituted methylenedioxyphenethylamine and substituted amphetamine classes of chemicals. As a free base, MDMA is a colorless oil insoluble in water.[14] The most common salt of MDMA is the hydrochloride salt;[14] pure MDMA hydrochloride is water-soluble and appears as a white or off-white powder or crystal.[14]
Synthesis
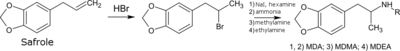
There are numerous methods available to synthesize MDMA via different intermediates.[224][225][226][227] The original MDMA synthesis described in Merck's patent involves brominating safrole to 1-(3,4-methylenedioxyphenyl)-2-bromopropane and then reacting this adduct with methylamine.[228][229] Most illicit MDMA is synthesized using MDP2P (3,4-methylenedioxyphenyl-2-propanone) as a precursor. MDP2P in turn is generally synthesized from piperonal, safrole or isosafrole.[230] One method is to isomerize safrole to isosafrole in the presence of a strong base, and then oxidize isosafrole to MDP2P. Another method uses the Wacker process to oxidize safrole directly to the MDP2P intermediate with a palladium catalyst. Once the MDP2P intermediate has been prepared, a reductive amination leads to racemic MDMA (an equal parts mixture of (R)-MDMA and (S)-MDMA). Relatively small quantities of essential oil are required to make large amounts of MDMA. The essential oil of Ocotea cymbarum, for example, typically contains between 80 and 94% safrole. This allows 500 mL of the oil to produce between 150 and 340 grams of MDMA.[231]
Detection in body fluids
MDMA and MDA may be quantitated in blood, plasma or urine to monitor for use, confirm a diagnosis of poisoning or assist in the forensic investigation of a traffic or other criminal violation or a sudden death. Some drug abuse screening programs rely on hair, saliva, or sweat as specimens. Most commercial amphetamine immunoassay screening tests cross-react significantly with MDMA or its major metabolites, but chromatographic techniques can easily distinguish and separately measure each of these substances. The concentrations of MDA in the blood or urine of a person who has taken only MDMA are, in general, less than 10% those of the parent drug.[220][232][233]
History
Early research and use
MDMA was first synthesized and patented in 1912 by Merck chemist Anton Köllisch.[234][235] At the time, Merck was interested in developing substances that stopped abnormal bleeding. Merck wanted to avoid an existing patent held by Bayer for one such compound: hydrastinine. Köllisch developed a preparation of a hydrastinine analogue, methylhydrastinine, at the request of fellow lab members, Walther Beckh and Otto Wolfes. MDMA (called methylsafrylamin, safrylmethylamin or N-Methyl-a-Methylhomopiperonylamin in Merck laboratory reports) was an intermediate compound in the synthesis of methylhydrastinine. Merck was not interested in MDMA itself at the time.[235] On 24 December 1912, Merck filed two patent applications that described the synthesis and some chemical properties of MDMA[236] and its subsequent conversion to methylhydrastinine.[237] Merck records indicate its researchers returned to the compound sporadically. A 1920 Merck patent describes a chemical modification to MDMA.[234][238]
MDMA's analogue 3,4-methylenedioxyamphetamine (MDA) was first synthesized in 1910 as a derivative of adrenaline.[234] Gordon A. Alles, the discoverer of the psychoactive effects of amphetamine, also discovered the psychoactive effects of MDA in 1930 in a self-experiment in which he administered a high dose (126 mg) to himself.[234][239][240] However, he did not subsequently describe these effects until 1959.[241][239][240] MDA was later tested as an appetite suppressant by Smith, Kline & French and for other uses by other groups in the 1950s.[234] In relation to the preceding, the psychoactive effects of MDA were discovered well before those of MDMA.[234][241]
In 1927, Max Oberlin studied the pharmacology of MDMA while searching for substances with effects similar to adrenaline or ephedrine, the latter being structurally similar to MDMA. Compared to ephedrine, Oberlin observed that it had similar effects on vascular smooth muscle tissue, stronger effects at the uterus, and no "local effect at the eye". MDMA was also found to have effects on blood sugar levels comparable to high doses of ephedrine. Oberlin concluded that the effects of MDMA were not limited to the sympathetic nervous system. Research was stopped "particularly due to a strong price increase of safrylmethylamine", which was still used as an intermediate in methylhydrastinine synthesis. Albert van Schoor performed simple toxicological tests with the drug in 1952, most likely while researching new stimulants or circulatory medications. After pharmacological studies, research on MDMA was not continued. In 1959, Wolfgang Fruhstorfer synthesized MDMA for pharmacological testing while researching stimulants. It is unclear if Fruhstorfer investigated the effects of MDMA in humans.[235]
Outside of Merck, other researchers began to investigate MDMA. In 1953 and 1954, the United States Army commissioned a study of toxicity and behavioral effects in animals injected with mescaline and several analogues, including MDMA. Conducted at the University of Michigan in Ann Arbor, these investigations were declassified in October 1969 and published in 1973.[242][241] A 1960 Polish paper by Biniecki and Krajewski describing the synthesis of MDMA as an intermediate was the first published scientific paper on the substance.[235][241][243]
MDA appeared as a recreational drug in the mid-1960s.[234] MDMA may have been in non-medical use in the western United States in 1968.[234][244] An August 1970 report at a meeting of crime laboratory chemists indicates MDMA was being used recreationally in the Chicago area by 1970.[241][245] MDMA likely emerged as a substitute for MDA,[246] a drug at the time popular among users of psychedelics[247] which was made a Schedule 1 controlled substance in the United States in 1970.[248][249]
Shulgin's research

American chemist and psychopharmacologist Alexander Shulgin reported he synthesized MDMA in 1965 while researching methylenedioxy compounds at Dow Chemical Company, but did not test the psychoactivity of the compound at this time. Around 1970, Shulgin sent instructions for N-methylated MDA (MDMA) synthesis to the founder of a Los Angeles chemical company who had requested them. This individual later provided these instructions to a client in the Midwest. Shulgin may have suspected he played a role in the emergence of MDMA in Chicago.[241]
Shulgin first heard of the psychoactive effects of N-methylated MDA around 1975 from a young student who reported "amphetamine-like content".[241] Around 30 May 1976, Shulgin again heard about the effects of N-methylated MDA,[241] this time from a graduate student in a medicinal chemistry group he advised at San Francisco State University[247][250] who directed him to the University of Michigan study.[251] She and two close friends had consumed 100 mg of MDMA and reported positive emotional experiences.[241] Following the self-trials of a colleague at the University of San Francisco, Shulgin synthesized MDMA and tried it himself in September and October 1976.[241][247] Shulgin first reported on MDMA in a presentation at a conference in Bethesda, Maryland in December 1976.[241] In 1978, he and David E. Nichols published a report on the drug's psychoactive effect in humans.[234] They described MDMA as inducing "an easily controlled altered state of consciousness with emotional and sensual overtones" comparable "to marijuana, to psilocybin devoid of the hallucinatory component, or to low levels of MDA".[252]
While not finding his own experiences with MDMA particularly powerful,[251][253] Shulgin was impressed with the drug's disinhibiting effects and thought it could be useful in therapy.[253] Believing MDMA allowed users to strip away habits and perceive the world clearly, Shulgin called the drug window.[251][254] Shulgin occasionally used MDMA for relaxation, referring to it as "my low-calorie martini", and gave the drug to friends, researchers, and others who he thought could benefit from it.[251] One such person was Leo Zeff, a psychotherapist who had been known to use psychedelic substances in his practice. When he tried the drug in 1977, Zeff was impressed with the effects of MDMA and came out of his semi-retirement to promote its use in therapy. Over the following years, Zeff traveled around the United States and occasionally to Europe, eventually training an estimated four thousand psychotherapists in the therapeutic use of MDMA.[253][255] Zeff named the drug Adam, believing it put users in a state of primordial innocence.[247]
Psychotherapists who used MDMA believed the drug eliminated the typical fear response and increased communication. Sessions were usually held in the home of the patient or the therapist. The role of the therapist was minimized in favor of patient self-discovery accompanied by MDMA induced feelings of empathy. Depression, substance use disorders, relationship problems, premenstrual syndrome, and autism were among several psychiatric disorders MDMA assisted therapy was reported to treat.[249] According to psychiatrist George Greer, therapists who used MDMA in their practice were impressed by the results. Anecdotally, MDMA was said to greatly accelerate therapy.[253] According to David Nutt, MDMA was widely used in the western US in couples counseling, and was called empathy. Only later was the term ecstasy used for it, coinciding with rising opposition to its use.[256][257]
Rising recreational use
In the late 1970s and early 1980s, "Adam" spread through personal networks of psychotherapists, psychiatrists, users of psychedelics, and yuppies. Hoping MDMA could avoid criminalization like LSD and mescaline, psychotherapists and experimenters attempted to limit the spread of MDMA and information about it while conducting informal research.[249][258] Early MDMA distributors were deterred from large scale operations by the threat of possible legislation.[259] Between the 1970s and the mid-1980s, this network of MDMA users consumed an estimated 500,000 doses.[5][260]
A small recreational market for MDMA developed by the late 1970s,[261] consuming perhaps 10,000 doses in 1976.[248] By the early 1980s MDMA was being used in Boston and New York City nightclubs such as Studio 54 and Paradise Garage.[262][263] Into the early 1980s, as the recreational market slowly expanded, production of MDMA was dominated by a small group of therapeutically minded Boston chemists. Having commenced production in 1976, this "Boston Group" did not keep up with growing demand and shortages frequently occurred.[259]
Perceiving a business opportunity, Michael Clegg, the Southwest distributor for the Boston Group, started his own "Texas Group" backed financially by Texas friends.[259][264] In 1981,[259] Clegg had coined "Ecstasy" as a slang term for MDMA to increase its marketability.[254][258] Starting in 1983,[259] the Texas Group mass-produced MDMA in a Texas lab[258] or imported it from California[254] and marketed tablets using pyramid sales structures and toll-free numbers.[260] MDMA could be purchased via credit card and taxes were paid on sales.[259] Under the brand name "Sassyfras", MDMA tablets were sold in brown bottles.[258] The Texas Group advertised "Ecstasy parties" at bars and discos, describing MDMA as a "fun drug" and "good to dance to".[259] MDMA was openly distributed in Austin and Dallas–Fort Worth area bars and nightclubs, becoming popular with yuppies, college students, and gays.[246][259][260]
Recreational use also increased after several cocaine dealers switched to distributing MDMA following experiences with the drug.[260] A California laboratory that analyzed confidentially submitted drug samples first detected MDMA in 1975. Over the following years the number of MDMA samples increased, eventually exceeding the number of MDA samples in the early 1980s.[265][266] By the mid-1980s, MDMA use had spread to colleges around the United States.[259]: 33
Media attention and scheduling
United States

In an early media report on MDMA published in 1982, a Drug Enforcement Administration (DEA) spokesman stated the agency would ban the drug if enough evidence for abuse could be found.[259] By mid-1984, MDMA use was becoming more noticed. Bill Mandel reported on "Adam" in a 10 June San Francisco Chronicle article, but misidentified the drug as methyloxymethylenedioxyamphetamine (MMDA). In the next month, the World Health Organization identified MDMA as the only substance out of twenty phenethylamines to be seized a significant number of times.[258]
After a year of planning and data collection, MDMA was proposed for scheduling by the DEA on 27 July 1984, with a request for comments and objections.[258][267] The DEA was surprised when a number of psychiatrists, psychotherapists, and researchers objected to the proposed scheduling and requested a hearing.[249] In a Newsweek article published the next year, a DEA pharmacologist stated that the agency had been unaware of its use among psychiatrists.[268] An initial hearing was held on 1 February 1985 at the DEA offices in Washington, D.C., with administrative law judge Francis L. Young presiding.[258] It was decided there to hold three more hearings that year: Los Angeles on 10 June, Kansas City, Missouri on 10–11 July, and Washington, D.C., on 8–11 October.[249][258]
Sensational media attention was given to the proposed criminalization and the reaction of MDMA proponents, effectively advertising the drug.[249] In response to the proposed scheduling, the Texas Group increased production from 1985 estimates of 30,000 tablets a month to as many as 8,000 per day, potentially making two million ecstasy tablets in the months before MDMA was made illegal.[269] By some estimates the Texas Group distributed 500,000 tablets per month in Dallas alone.[254] According to one participant in an ethnographic study, the Texas Group produced more MDMA in eighteen months than all other distribution networks combined across their entire histories.[259] By May 1985, MDMA use was widespread in California, Texas, southern Florida, and the northeastern United States.[244][270] According to the DEA there was evidence of use in twenty-eight states[271] and Canada.[244] Urged by Senator Lloyd Bentsen, the DEA announced an emergency Schedule I classification of MDMA on 31 May 1985. The agency cited increased distribution in Texas, escalating street use, and new evidence of MDA (an analog of MDMA) neurotoxicity as reasons for the emergency measure.[270][272][273] The ban took effect one month later on 1 July 1985[269] in the midst of Nancy Reagan's "Just Say No" campaign.[274][275]
As a result of several expert witnesses testifying that MDMA had an accepted medical usage, the administrative law judge presiding over the hearings recommended that MDMA be classified as a Schedule III substance. Despite this, DEA administrator John C. Lawn overruled and classified the drug as Schedule I.[249][276] Harvard psychiatrist Lester Grinspoon then sued the DEA, claiming that the DEA had ignored the medical uses of MDMA, and the federal court sided with Grinspoon, calling Lawn's argument "strained" and "unpersuasive", and vacated MDMA's Schedule I status.[277] Despite this, less than a month later Lawn reviewed the evidence and reclassified MDMA as Schedule I again, claiming that the expert testimony of several psychiatrists claiming over 200 cases where MDMA had been used in a therapeutic context with positive results could be dismissed because they were not published in medical journals.[249] In 2017, the FDA granted breakthrough therapy designation for its use with psychotherapy for PTSD. However, this designation has been questioned and problematized.[278]
United Nations
While engaged in scheduling debates in the United States, the DEA also pushed for international scheduling.[269] In 1985, the World Health Organization's Expert Committee on Drug Dependence recommended that MDMA be placed in Schedule I of the 1971 United Nations Convention on Psychotropic Substances. The committee made this recommendation on the basis of the pharmacological similarity of MDMA to previously scheduled drugs, reports of illicit trafficking in Canada, drug seizures in the United States, and lack of well-defined therapeutic use. While intrigued by reports of psychotherapeutic uses for the drug, the committee viewed the studies as lacking appropriate methodological design and encouraged further research. Committee chairman Paul Grof dissented, believing international control was not warranted at the time and a recommendation should await further therapeutic data.[279] The Commission on Narcotic Drugs added MDMA to Schedule I of the convention on 11 February 1986.[280]
Post-scheduling

File:Ecstasy - Is it Really the Dream Drug.ogv
The use of MDMA in Texas clubs declined rapidly after criminalization, but by 1991, the drug became popular among young middle-class whites and in nightclubs.[259] In 1985, MDMA use became associated with acid house on the Spanish island of Ibiza.[259]: 50 [281] Thereafter, in the late 1980s, the drug spread alongside rave culture to the United Kingdom and then to other European and American cities.[259]: 50 Illicit MDMA use became increasingly widespread among young adults in universities and later, in high schools. Since the mid-1990s, MDMA has become the most widely used amphetamine-type drug by college students and teenagers.[282]: 1080 MDMA became one of the four most widely used illicit drugs in the US, along with cocaine, heroin, and cannabis.[254] According to some estimates as of 2004, only marijuana attracts more first time users in the United States.[254]
After MDMA was criminalized, most medical use stopped, although some therapists continued to prescribe the drug illegally. Later,[when?] Charles Grob initiated an ascending-dose safety study in healthy volunteers. Subsequent FDA-approved MDMA studies in humans have taken place in the United States in Detroit (Wayne State University), Chicago (University of Chicago), San Francisco (UCSF and California Pacific Medical Center), Baltimore (NIDA–NIH Intramural Program), and South Carolina. Studies have also been conducted in Switzerland (University Hospital of Psychiatry, Zürich), the Netherlands (Maastricht University), and Spain (Universitat Autònoma de Barcelona).[283]
"Molly", short for 'molecule', was recognized as a slang term for crystalline or powder MDMA in the 2000s.[284][285]
In 2010, the BBC reported that use of MDMA had decreased in the UK in previous years. This may be due to increased seizures during use and decreased production of the precursor chemicals used to manufacture MDMA. Unwitting substitution with other drugs, such as mephedrone and methamphetamine,[286] as well as legal alternatives to MDMA, such as BZP, MDPV, and methylone, are also thought to have contributed to its decrease in popularity.[287]
In 2017, it was found that some pills being sold as MDMA contained pentylone, which can cause very unpleasant agitation and paranoia.[288]
According to David Nutt, when safrole was restricted by the United Nations in order to reduce the supply of MDMA, producers in China began using anethole instead, but this gives para-methoxyamphetamine (PMA, also known as "Dr Death"), which is much more toxic than MDMA and can cause overheating, muscle spasms, seizures, unconsciousness, and death. People wanting MDMA are sometimes sold PMA instead.[256]
In 2025, the BBC reported on a study of 650 survivors from the Nova music festival massacre. Two-thirds were under the influence of recreational drugs (MDMA, LSD, marijuana or psilocybin) when Hamas attacked the festival on October 7, 2023. MDMA appeared to have a protective effect against later problems with sleeping and emotional distress.[289][290]
Society and culture
| Substance | Best estimate |
Low estimate |
High estimate |
|---|---|---|---|
| Amphetamine- type stimulants |
34.16 | 13.42 | 55.24 |
| Cannabis | 192.15 | 165.76 | 234.06 |
| Cocaine | 18.20 | 13.87 | 22.85 |
| Ecstasy | 20.57 | 8.99 | 32.34 |
| Opiates | 19.38 | 13.80 | 26.15 |
| Opioids | 34.26 | 27.01 | 44.54 |
Legal status
MDMA is legally controlled in most of the world under the UN Convention on Psychotropic Substances and other international agreements, although exceptions exist for research and limited medical use. In general, the unlicensed use, sale or manufacture of MDMA are all criminal offences.
Australia
In Australia, MDMA was rescheduled on 1 July 2023 as a schedule 8 substance (available on prescription) when used in the treatment of PTSD, while remaining a schedule 9 substance (prohibited) for all other uses. For the treatment of PTSD, MDMA can only be prescribed by psychiatrists with specific training and authorisation.[292] In 1986, MDMA was declared an illegal substance because of its allegedly harmful effects and potential for misuse.[293] Any non-authorised sale, use or manufacture is strictly prohibited by law. Permits for research uses on humans must be approved by a recognized ethics committee on human research.
In Western Australia under the Misuse of Drugs Act 1981 4.0g of MDMA is the amount required determining a court of trial, 2.0g is considered a presumption with intent to sell or supply and 28.0g is considered trafficking under Australian law.[294]
The Australian Capital Territory passed legislation to decriminalise the possession of small amounts of MDMA, which took effect in October 2023.[295][296]
Canada
In Canada, MDMA is listed as a Schedule 1[297] as it is an analogue of amphetamine.[298] The Controlled Drugs and Substances Act was updated as a result of the Safe Streets and Communities Act changing amphetamines from Schedule III to Schedule I in March 2012. In 2022, the federal government granted British Columbia a 3-year exemption, legalizing the possession of up to 2.5 grams (0.088 oz) of MDMA in the province from February 2023 until February 2026.[299][300]
Finland
Scheduled in the "government decree on substances, preparations and plants considered to be narcotic drugs".[301] Ecstasy is considered a very dangerous illegal drug.[302]
Netherlands
In 2024, a Dutch state commission issued a report advocating for MDMA to be made available to patients with PTSD.[303]
In June 2011, the Expert Committee on the List (Expertcommissie Lijstensystematiek Opiumwet) issued a report which discussed the evidence for harm and the legal status of MDMA, arguing in favor of maintaining it on List I.[304][305][306]
United Kingdom
In the United Kingdom, MDMA was made illegal in 1977 by a modification order to the existing Misuse of Drugs Act 1971. Although MDMA was not named explicitly in this legislation, the order extended the definition of Class A drugs to include various ring-substituted phenethylamines.[307][308] The drug is therefore illegal to sell, buy, or possess without a licence in the UK. Penalties include a maximum of seven years and/or unlimited fine for possession; life and/or unlimited fine for production or trafficking.
Some researchers such as David Nutt have criticized the scheduling of MDMA, which he determined to be a relatively harmless drug.[309][310] An editorial he wrote in the Journal of Psychopharmacology, where he compared the risk of harm for horse riding (1 adverse event in 350) to that of ecstasy (1 in 10,000) resulted in his dismissal, leading to the resignation of several of his colleagues from the ACMD.[311]
United States
In the United States, MDMA is listed in Schedule I of the Controlled Substances Act.[312] In a 2011 federal court hearing, the American Civil Liberties Union successfully argued that the sentencing guideline for MDMA/ecstasy is based on outdated science, leading to excessive prison sentences.[313] Other courts have upheld the sentencing guidelines. The United States District Court for the Eastern District of Tennessee explained its ruling by noting that "an individual federal district court judge simply cannot marshal resources akin to those available to the Commission for tackling the manifold issues involved with determining a proper drug equivalency."[304]
Demographics
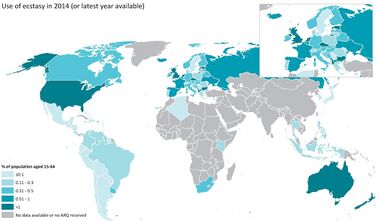
In 2014, 3.5% of 18-to-25-year-olds had used MDMA in the United States.[36] In the European Union as of 2018, 4.1% of adults (15–64 years old) have used MDMA at least once in their life, and 0.8% had used it in the last year.[314] Among young adults, 1.8% had used MDMA in the last year.[314]
In Europe, an estimated 37% of regular club-goers aged 14 to 35 used MDMA in the past year according to the 2015 European Drug report.[36] The highest one-year prevalence of MDMA use in Germany in 2012 was 1.7% among people aged 25 to 29 compared with a population average of 0.4%.[36] Among adolescent users in the United States between 1999 and 2008, girls were more likely to use MDMA than boys.[315]
Economics
Europe
In 2008 the European Monitoring Centre for Drugs and Drug Addiction noted that although there were some reports of tablets being sold for as little as €1, most countries in Europe then reported typical retail prices in the range of €3 to €9 per tablet, typically containing 25–65 mg of MDMA.[316] By 2014 the EMCDDA reported that the range was more usually between €5 and €10 per tablet, typically containing 57–102 mg of MDMA, although MDMA in powder form was becoming more common.[317]
North America
The United Nations Office on Drugs and Crime stated in its 2014 World Drug Report that US ecstasy retail prices range from US$1 to $70 per pill, or from $15,000 to $32,000 per kilogram.[318] A new research area named Drug Intelligence aims to automatically monitor distribution networks based on image processing and machine learning techniques, in which an Ecstasy pill picture is analyzed to detect correlations among different production batches.[319] These novel techniques allow police scientists to facilitate the monitoring of illicit distribution networks.
As of October 2015[update], most of the MDMA in the United States is produced in British Columbia, Canada and imported by Canada-based Asian transnational criminal organizations.[46] The market for MDMA in the United States is relatively small compared to methamphetamine, cocaine, and heroin.[46] In the United States, about 0.9 million people used ecstasy in 2010.[7]
Australia
MDMA is particularly expensive in Australia, costing A$15–A$30 per tablet. In terms of purity data for Australian MDMA, the average is around 34%, ranging from less than 1% to about 85%. The majority of tablets contain 70–85 mg of MDMA. Most MDMA enters Australia from the Netherlands, the UK, Asia, and the US.[320]
Corporate logos on pills
A number of ecstasy manufacturers brand their pills with a logo, often that of an unrelated corporation.[321] Some pills depict logos of products or media popular with children, such as Shaun the Sheep.[322]
Research
MDMA-assisted psychotherapy shows promising efficacy and a generally tolerable safety profile for treating PTSD, with meta-analyses indicating symptom reduction, though careful dosing and controlled therapeutic settings are essential to minimize risks.[19][20]
MDMA is being investigated as a potential treatment for social impairments in autism.[323]
The British critical psychiatrist Joanna Moncrieff has critiqued the use and study of MDMA and related drugs like psychedelics for treatment of psychiatric disorders, highlighting concerns including excessive hype around these drugs, blurred lines between medical and recreational use, flawed clinical trial findings, financial conflicts of interest, strong expectancy effects and large placebo responses, short-term benefits over placebo, and their potential for adverse effects, among others.[324]
See also
- I Feel Love: MDMA and the Quest for Connection in a Fractured World
- Lykos Therapeutics
- Multidisciplinary Association for Psychedelic Studies (MAPS)
References
- ↑ "There's something about Molly: The underresearched yet popular powder form of ecstasy in the United States". Substance Abuse 38 (1): 15–17. 2016-12-07. doi:10.1080/08897077.2016.1267070. PMID 27925866.
- ↑ "Hva er tryggest av molly og ecstasy?" (in no). Norwegian Directorate for Children, Youth and Family Affairs. 2020-12-14. https://www.ung.no/oss/rusmidler/462397.html. "MDMA er virkestoffet i både Molly-krystaller og Ecstasy-tabletter. (MDMA is the active substance in both Molly crystals and Ecstasy tablets)"
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 "Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine". ACS Chemical Neuroscience 9 (10): 2408–2427. October 2018. doi:10.1021/acschemneuro.8b00155. PMID 30001118. PMC 6197894. https://shaunlacob.com/wp-content/uploads/2020/12/DC-MDMA.pdf.
- ↑ "The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy")". Pharmacological Reviews 55 (3): 463–508. September 2003. doi:10.1124/pr.55.3.3. PMID 12869661.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 "3,4-methylenedioxymethamphetamine (MDMA): current perspectives". Substance Abuse and Rehabilitation 4: 83–99. 2013. doi:10.2147/SAR.S37258. PMID 24648791.
- ↑ "The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents". Addiction (Abingdon, England) 101 (9): 1241–1245. August 2006. doi:10.1111/j.1360-0443.2006.01511.x. PMID 16911722. http://www.thedea.org/docs/2006_Freudenmann_22846_1.pdf. Retrieved 23 May 2019. "Although MDMA was, in fact, first synthesized at Merck in 1912, it was not tested pharmacologically because it was only an unimportant precursor in a new synthesis for haemostatic substances.".
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 "MDMA". Drugsite Trust. 18 May 2014. https://www.drugs.com/illicit/mdma.html.
- ↑ 8.0 8.1 8.2 8.3 "DrugFacts: MDMA (Ecstasy/Molly)". February 2016. https://www.drugabuse.gov/publications/drugfacts/mdma-ecstasymolly.
- ↑ Neuroscience of Psychoactive Substance Use and Dependence. World Health Organization. 2004. pp. 97–. ISBN 978-92-4-156235-5. https://books.google.com/books?id=G9OhG-dZdAwC&pg=PA97.
- ↑ World Drug Report 2018. United Nations. June 2018. p. 7. ISBN 978-92-1-148304-8. https://www.unodc.org/wdr2018/prelaunch/WDR18_Booklet_1_EXSUM.pdf. Retrieved 14 July 2018.
- ↑ "MDMA (Ecstasy/Molly)". https://www.drugabuse.gov/drugs-abuse/mdma-ecstasymolly.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 "How MDMA's pharmacology and pharmacokinetics drive desired effects and harms". Journal of Clinical Pharmacology 54 (3): 245–252. March 2014. doi:10.1002/jcph.266. PMID 24431106.
- ↑ "Pharmacological Effects of MDMA in Man". Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs. Springer Netherlands. 28 July 2009. pp. 151–160. doi:10.1007/978-90-481-2448-0_24. ISBN 978-90-481-2448-0.
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 Cite error: Invalid
<ref>tag; no text was provided for refs namedEU2015 - ↑ (in en) Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs: A comprehensive review on their mode of action, treatment of abuse and intoxication. Springer Science & Business Media. 2009. p. 147. ISBN 978-90-481-2448-0. https://books.google.com/books?id=OTAlolM3XlwC&pg=PA147. Retrieved 12 May 2020.
- ↑ "Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms". Brain Research. Brain Research Reviews 42 (2): 155–168. May 2003. doi:10.1016/S0165-0173(03)00173-5. PMID 12738056.
- ↑ "Ecstasy as a Remedy for PTSD? You Probably Have Some Questions." (in en). The New York Times. 1 May 2018. https://www.nytimes.com/2018/05/01/us/ecstasy-molly-ptsd-mdma.html.
- ↑ Mental and neurological public health a global perspective (1st ed.). San Diego, CA: Academic Press/Elsevier. 2010. p. 57. ISBN 978-0-12-381527-9. https://books.google.com/books?id=8fihf3VWbcIC&pg=PA57.
- ↑ 19.0 19.1 "The Efficacy of MDMA (3,4-Methylenedioxymethamphetamine) for Post-traumatic Stress Disorder in Humans: A Systematic Review and Meta-Analysis". Cureus 13 (5). May 2021. doi:10.7759/cureus.15070. PMID 34150406.
- ↑ 20.0 20.1 "MDMA-Assisted Psychotherapy for Treatment of Posttraumatic Stress Disorder: A Systematic Review With Meta-Analysis". Journal of Clinical Pharmacology 62 (4): 463–471. April 2022. doi:10.1002/jcph.1995. PMID 34708874.
- ↑ "A Psychedelic Drug Passes a Big Test for PTSD Treatment". The New York Times. 3 May 2021. https://www.nytimes.com/2021/05/03/health/mdma-approval.html.
- ↑ "Subsection 56(1) class exemption for practitioners, agents, pharmacists, persons in charge of a hospital, hospital employees, and licensed dealers to conduct activities with psilocybin and MDMA in relation to a special access program authorization". Health Canada. 2022-01-05. https://www.canada.ca/en/health-canada/services/health-concerns/controlled-substances-precursor-chemicals/policy-regulations/policy-documents/subsection-56-1-class-exemption-conducting-activities-psilocybin-mdma-special-access-program-authorization.html.
- ↑ "Change to classification of psilocybin and MDMA to enable prescribing by authorised psychiatrists". 3 February 2023. https://www.tga.gov.au/news/media-releases/change-classification-psilocybin-and-mdma-enable-prescribing-authorised-psychiatrists.
- ↑ 24.00 24.01 24.02 24.03 24.04 24.05 24.06 24.07 24.08 24.09 24.10 24.11 24.12 24.13 24.14 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid22392347 - ↑ Generation Ecstasy: Into the World of Techno and Rave Culture. Routledge. 1999. p. 81. ISBN 978-0-415-92373-6. https://books.google.com/books?id=tGaRJiXe74UC&q=Generation+Ecstasy+MDMA&pg=PA81. Retrieved 13 October 2020.
- ↑ 26.0 26.1 Addictions: a comprehensive guidebook (Second ed.). Oxford: Oxford University Press. 2013. p. 299. ISBN 978-0-19-975366-6. https://books.google.com/books?id=MUYfAQAAQBAJ&pg=PA299. Retrieved 11 January 2017.
- ↑ 27.0 27.1 "Co-use of MDMA with psilocybin/LSD may buffer against challenging experiences and enhance positive experiences". Scientific Reports 13 (1). August 2023. doi:10.1038/s41598-023-40856-5. PMID 37608057. Bibcode: 2023NatSR..1313645Z.
- ↑ "Nexus Flipping: What Happens When You Combine MDMA and 2C-B" (in en-US). 2024-02-16. https://doubleblindmag.com/nexus-flipping/.
- ↑ 29.0 29.1 "Duloxetine inhibits effects of MDMA ("ecstasy") in vitro and in humans in a randomized placebo-controlled laboratory study". PLOS ONE 7 (5). 2012-05-04. doi:10.1371/journal.pone.0036476. PMID 22574166. Bibcode: 2012PLoSO...736476H. "Fig. 7 shows the mean PD effects of MDMA plotted against simultaneous plasma concentrations at the different time points (hysteresis loops). The increases in "any drug effect" (Fig. 7a) and MAP (Fig. 7b) returned to baseline within 6 h when MDMA concentrations were still high. This clockwise hysteresis indicates that a smaller MDMA effect was seen at a given plasma concentration later in time, indicating rapid acute pharmacodynamic tolerance, which was similarly described for cocaine [33]. [...] Figure 7. Pharmacokinetic-pharmacodynamic (PK-PD) relationship. MDMA effects are plotted against simultaneous MDMA plasma concentrations (a, b). The time of sampling is noted next to each point in minutes or hours after MDMA administration. The clockwise hysteresis indicates acute tolerance to the effects of MDMA.".
- ↑ "Warning against co-administration of 3,4-methylenedioxymethamphetamine (MDMA) with methamphetamine from the perspective of pharmacokinetic and pharmacodynamic evaluations in rat brain". European Journal of Pharmaceutical Sciences 49 (1): 57–64. April 2013. doi:10.1016/j.ejps.2013.01.014. PMID 23395913.
- ↑ "Ethanol increases the distribution of MDMA to the rat brain: possible implications in the ethanol-induced potentiation of the psychostimulant effects of MDMA". The International Journal of Neuropsychopharmacology 12 (6): 749–759. July 2009. doi:10.1017/s1461145708009693. PMID 19046482.
- ↑ "Ecstasy: pharmacodynamic and pharmacokinetic interactions". Psychosomatics 45 (1): 84–87. 2004-03-01. doi:10.1176/appi.psy.45.1.84. PMID 14709765.
- ↑ "Clinically Relevant Drug-Drug Interactions in Primary Care". American Family Physician 99 (9): 558–564. May 2019. PMID 31038898.
- ↑ "Making a medicine out of MDMA". The British Journal of Psychiatry 206 (1): 4–6. January 2015. doi:10.1192/bjp.bp.114.152751. PMID 25561485.
- ↑ "Behavioral and Stereological Analysis of the Effects of Intermittent Feeding Diet on the Orally Administrated MDMA ("ecstasy") in Mice". Innovations in Clinical Neuroscience 14 (1–2): 40–52. 1 February 2017. PMID 28386520. "MDMA is listed as a Schedule 1 drug by the United States Drug Enforcement Agency, meaning that currently there are no accepted medical uses for MDMA in the United States, there is a lack of accepted safety for use under medical supervision, and there is a high potential for abuse.".
- ↑ 36.00 36.01 36.02 36.03 36.04 36.05 36.06 36.07 36.08 36.09 36.10 36.11 36.12 36.13 36.14 36.15 36.16 36.17 36.18 36.19 36.20 36.21 36.22 36.23 36.24 36.25 36.26 36.27 36.28 36.29 36.30 36.31 36.32 Cite error: Invalid
<ref>tag; no text was provided for refs namedBetzler_2017 - ↑ "Ecstacy: a review of MDMA and MDA". International Journal of Psychiatry in Medicine 16 (4): 359–372. 1986. doi:10.2190/dcrp-u22m-aumd-d84h. PMID 2881902.
- ↑ "Ecstasy could be 'breakthrough' therapy for soldiers, others suffering from PTSD" (in en-US). The Washington Post. 6 August 2017. https://www.washingtonpost.com/national/health-science/ecstasy-could-be-breakthrough-therapy-for-soldiers-others-suffering-from-ptsd/2017/08/26/009314ca-842f-11e7-b359-15a3617c767b_story.html.
- ↑ "All clear for the decisive trial of ecstasy in PTSD patients" (in en). 26 August 2017. https://www.science.org/content/article/all-clear-decisive-trial-ecstasy-ptsd-patients.
- ↑ "Culture, context, and ethics in the therapeutic use of hallucinogens: Psychedelics as active super-placebos?". Transcultural Psychiatry 59 (5): 571–578. October 2022. doi:10.1177/13634615221131465. PMID 36263513.
- ↑ "Pharmacological, neural, and psychological mechanisms underlying psychedelics: A critical review". Neuroscience and Biobehavioral Reviews 140. September 2022. doi:10.1016/j.neubiorev.2022.104793. PMID 35878791. "In addition, the strong prior expectations that many people have about psychedelics directly contribute to the psychedelic experience and as a consequence it has been suggested that psychedelics may act as a 'super-placebo' (Hartogsohn, 2016). Specifically, strong prior expectations (e.g., that a specific intervention will likely trigger a mystical experience) will increase the likelihood of having e.g., a mystical-type experience (Maij et al., 2019), and this placebo-effect is further boosted by the psychedelic-induced suggestibility.".
- ↑ "The Agony and Ecstasy of God's path". Council on Spiritual Practices (CSP). 29 July 1995. http://csp.org/practices/entheogens/docs/saunders-ecstasy_rel.html.
- ↑ "New age seekers: MDMA use as an adjunct to spiritual pursuit". Journal of Psychoactive Drugs 23 (3): 261–270. 1991. doi:10.1080/02791072.1991.10471587. PMID 1685513. http://research.lycaeum.org/researchpdfs/1991_watson_1.pdf. Retrieved 28 April 2024.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedLuciano_2014 - ↑ 45.0 45.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedDrugFacts - ↑ 46.0 46.1 46.2 46.3 "MDMA (3,4-Methylenedioxymethamphetamine)". 2015 National Drug Threat Assessment Summary. United States Department of Justice, Drug Enforcement Administration. October 2015. pp. 85–88. http://www.dea.gov/docs/2015%20NDTA%20Report.pdf. Retrieved 10 April 2016.
- ↑ 47.0 47.1 Molly Madness. Drugs, Inc. (TV documentary). National Geographic Channel. 13 August 2014. ASIN B00LIC368M.
- ↑ 48.0 48.1 Manic Molly. Drugs, Inc. (TV documentary). National Geographic Channel. 10 December 2014. ASIN B00LIC368M.
- ↑ "Man arrested for possession of ecstasy tablets shaped like Wario". Nintendo Enthusiast. 20 June 2019. https://www.nintendoenthusiast.com/man-arrested-for-possession-of-ecstasy-tablets-shaped-like-wario/.
- ↑ "Groesbeck: Students caught with deceptively shaped Ecstasy pills". KWTX. 31 October 2019. https://www.kwtx.com/content/news/Students-caught-with-deceptively-shaped-Ecstasy-pills-at-local-school-564188221.html.
- ↑ 51.00 51.01 51.02 51.03 51.04 51.05 51.06 51.07 51.08 51.09 51.10 51.11 51.12 51.13 51.14 51.15 51.16 51.17 51.18 "3,4-Methylenedioxymethamphetamine". National Library of Medicine. 28 August 2008. https://www.nlm.nih.gov/toxnet/index.html.
- ↑ "Gender differences in the subjective effects of MDMA". Psychopharmacology 154 (2): 161–168. March 2001. doi:10.1007/s002130000648. PMID 11314678.
- ↑ 53.00 53.01 53.02 53.03 53.04 53.05 53.06 53.07 53.08 53.09 53.10 53.11 53.12 53.13 53.14 53.15 53.16 53.17 53.18 53.19 53.20 53.21 53.22 53.23 53.24 53.25 53.26 53.27 53.28 53.29 "Review article: amphetamines and related drugs of abuse". Emergency Medicine Australasia 20 (5): 391–402. October 2008. doi:10.1111/j.1742-6723.2008.01114.x. PMID 18973636.
- ↑ 54.0 54.1 "MDMA and the states of Consciousness". Eleusis 2: 3–9. 1995.
- ↑ "Intimate insight: MDMA changes how people talk about significant others". Journal of Psychopharmacology (Oxford, England) 29 (6): 669–677. June 2015. doi:10.1177/0269881115581962. PMID 25922420.
- ↑ 56.0 56.1 "Differential effects of MDMA and methylphenidate on social cognition". Journal of Psychopharmacology (Oxford, England) 28 (9): 847–856. September 2014. doi:10.1177/0269881114542454. PMID 25052243. http://edoc.unibas.ch/42235/1/20160316152928_56e96dc8bdaad.pdf. Retrieved 29 June 2019.
- ↑ "MDMA alters emotional processing and facilitates positive social interaction". Psychopharmacology 231 (21): 4219–4229. October 2014. doi:10.1007/s00213-014-3570-x. PMID 24728603.
- ↑ "What does MDMA feel like?". Ecstasy: The complete guide. A comprehensive look at the risks and benefits of MDMA.. Rochester: Park Street Press. 2001.
- ↑ "Psychedelic, Psychoactive, and Addictive Drugs and States of Consciousness". Mind-Altering Drugs: The Science of Subjective Experience. New York: Oxford University. 2005. http://www.greenearthfound.org/write/psychedelic.html. Retrieved 8 October 2017.
- ↑ 60.0 60.1 60.2 60.3 "The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals". Neuroscience and Biobehavioral Reviews 57: 433–446. October 2015. doi:10.1016/j.neubiorev.2015.08.016. PMID 26408071.
- ↑ 61.0 61.1 61.2 61.3 61.4 61.5 61.6 "Recognising and managing acute hyponatraemia". Emergency Nurse 21 (9): 32–6; quiz 37. February 2014. doi:10.7748/en2014.02.21.9.32.e1128. PMID 24494770.
- ↑ "Drug use, sexual risk behaviour and sexually transmitted infections among swingers: a cross-sectional study in The Netherlands". Sexually Transmitted Infections 91 (1): 31–36. February 2015. doi:10.1136/sextrans-2014-051626. PMID 25342812. "It is known that some recreational drugs (eg, MDMA or GHB) may hamper the potential to ejaculate or maintain an erection.".
- ↑ "MDMA Toxicity: Background, Pathophysiology, Epidemiology". 25 March 2015. http://emedicine.medscape.com/article/821572-overview#showall.
- ↑ "13. MDMA and LSD". Drug Abuse and Addiction in Medical Illness: Causes, Consequences and Treatment. Springer Science & Business Media. 2012. p. 179. ISBN 978-1-4614-3375-0. https://books.google.com/books?id=yGeBj9U6Za8C.
- ↑ 65.0 65.1 65.2 65.3 65.4 65.5 65.6 65.7 "Functional Magnetic Resonance Imaging in Abstinent MDMA Users: A Review". Current Drug Abuse Reviews 8 (1): 15–25. 2015. doi:10.2174/1874473708666150303115833. PMID 25731754.
- ↑ 66.0 66.1 "Neuroimaging in moderate MDMA use: A systematic review". Neuroscience and Biobehavioral Reviews 62: 21–34. March 2016. doi:10.1016/j.neubiorev.2015.12.010. PMID 26746590.
- ↑ "Neurotoxicity of drugs of abuse--the case of methylenedioxyamphetamines (MDMA, ecstasy), and amphetamines". Dialogues in Clinical Neuroscience 11 (3): 305–317. 2009. doi:10.31887/DCNS.2009.11.3/egmayfrank. PMID 19877498.
- ↑ 68.0 68.1 "Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine". Life Sciences 97 (1): 37–44. February 2014. doi:10.1016/j.lfs.2013.07.014. PMID 23892199. "In contrast, MDMA produces damage to serotonergic, but not dopaminergic axon terminals in the striatum, hippocampus, and prefrontal cortex (Battaglia et al., 1987, O'Hearn et al., 1988). The damage associated with Meth and MDMA has been shown to persist for at least 2 years in rodents, non-human primates and humans (Seiden et al., 1988, Woolverton et al., 1989, McCann et al., 1998, Volkow et al., 2001a, McCann et al., 2005)".
- ↑ "Are ecstasy induced serotonergic alterations overestimated for the majority of users?". Journal of Psychopharmacology (Oxford, England) 32 (7): 741–748. July 2018. doi:10.1177/0269881118767646. PMID 29733742. "Given the dose-response relationship between MDMA exposure and SERT reductions and the statistically non-significant SERT binding differences for users with use levels similar to the majority of real-life users, it can be speculated that SERT levels may not be significantly affected for most recreational ecstasy users.".
- ↑ "Meta-analysis of molecular imaging of serotonin transporters in ecstasy/polydrug users". Neuroscience and Biobehavioral Reviews 63: 158–167. April 2016. doi:10.1016/j.neubiorev.2016.02.003. PMID 26855234.
- ↑ 71.0 71.1 71.2 71.3 "The potential dangers of using MDMA for psychotherapy". Journal of Psychoactive Drugs 46 (1): 37–43. 2014. doi:10.1080/02791072.2014.873690. PMID 24830184. https://www.researchgate.net/publication/262381558.
- ↑ "The harmful health effects of recreational ecstasy: a systematic review of observational evidence". Health Technology Assessment (Winchester, England) 13 (6): iii–iv, ix–xii, 1–315. January 2009. doi:10.3310/hta13050. PMID 19195429.
- ↑ "Verbal Memory Impairment in Polydrug Ecstasy Users: A Clinical Perspective". PLOS ONE 11 (2). 2016. doi:10.1371/journal.pone.0149438. PMID 26907605. Bibcode: 2016PLoSO..1149438K.
- ↑ "Ecstasy (MDMA) and memory function: a meta-analytic update". Human Psychopharmacology 22 (6): 381–388. August 2007. doi:10.1002/hup.857. PMID 17621368.
- ↑ "The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation". Frontiers in Pharmacology 3: 121. 2012. doi:10.3389/fphar.2012.00121. PMID 22754527.
- ↑ Human biology (10th ed.). Belmont, CA: Brooks/Cole Cengage Learning. 2014. ISBN 978-1-133-59916-6.
- ↑ "Methylenedioxymethamphetamine ('Ecstasy')-induced immunosuppression: a cause for concern?". British Journal of Pharmacology 161 (1): 17–32. September 2010. doi:10.1111/J.1476-5381.2010.00899.X. PMID 20718737.
- ↑ "Safety Pharmacology assessment of drugs with biased 5-HT(2B) receptor agonism mediating cardiac valvulopathy". Journal of Pharmacological and Toxicological Methods 69 (2): 150–161. 2014. doi:10.1016/j.vascn.2013.12.004. PMID 24361689.
- ↑ "Cardiovascular Concern of 5-HT2B Receptor and Recent Vistas in the Development of Its Antagonists". Cardiovascular & Hematological Disorders Drug Targets 17 (2): 86–104. 2017. doi:10.2174/1871529X17666170703115111. PMID 28676029.
- ↑ "Ecstasy (MDMA) Alters Cardiac Gene Expression and DNA Methylation: Implications for Circadian Rhythm Dysfunction in the Heart". Toxicological Sciences 148 (1): 183–191. November 2015. doi:10.1093/toxsci/kfv170. PMID 26251327.
- ↑ "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet (London, England) 369 (9566): 1047–1053. March 2007. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
Lay summary: "Scientists want new drug rankings". 23 March 2007. https://news.bbc.co.uk/2/hi/health/6474053.stm. - ↑ 82.0 82.1 "DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation". The Journal of Neuroscience 26 (36): 9196–9204. September 2006. doi:10.1523/JNEUROSCI.1124-06.2006. PMID 16957076.
- ↑ 83.0 83.1 "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews. Neuroscience 12 (11): 623–637. October 2011. doi:10.1038/nrn3111. PMID 21989194.
- ↑ Clinical Textbook of Addictive Disorders. Guilford Publications. 2016-05-12. p. 169. ISBN 978-1-4625-2169-2. https://books.google.com/books?id=88W_CwAAQBAJ&pg=PA171. "MDMA's addictive liability appears to be lower than that of other drugs of abuse...."
- ↑ "The Health Effect of Psychostimulants: A Literature Review". Pharmaceuticals (Basel, Switzerland) 3 (7): 2333–2361. July 2010. doi:10.3390/ph3072333. PMID 27713356. "It seems to present a smaller addiction potential than cocaine or methamphetamine.".
- ↑ Principles of addiction medicine. (4th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. 2009. p. 226. ISBN 978-0-7817-7477-2. https://books.google.com/books?id=j6GGBud8DXcC&pg=PA226. Retrieved 11 January 2017. "MDA and MDMA are less reinforcing than amphetamine..."
- ↑ 87.0 87.1 "The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, 'Ecstasy'), methamphetamine and D-amphetamine". Biological Chemistry 392 (1–2): 103–115. January 2011. doi:10.1515/BC.2011.016. PMID 21194370. "...approximately 15% of routine MDMA users recently fit the diagnostic criteria for MDMA dependence according to the Diagnostic and Statistical Manual, fourth edition/DSMIV.".
- ↑ Clinical Textbook of Addictive Disorders. Guilford Publications. 2016-05-12. p. 171. ISBN 978-1-4625-2169-2. https://books.google.com/books?id=88W_CwAAQBAJ&q=mdma+addiction&pg=PA171. Retrieved 13 October 2020. "There are no known pharmacological treatments for MDMA addiction."
- ↑ "Methods for detecting long-term CNS dysfunction after prenatal exposure to neurotoxins". Drug and Chemical Toxicology 20 (4): 387–399. November 1997. doi:10.3109/01480549709003895. PMID 9433666.
- ↑ 90.0 90.1 "Toxicity of ecstasy (MDMA) towards embryonic stem cell-derived cardiac and neural cells". Toxicology in Vitro 24 (4): 1133–1138. June 2010. doi:10.1016/j.tiv.2010.03.005. PMID 20230888. Bibcode: 2010ToxVi..24.1133M. "In summary, MDMA is a moderate teratogen that could influence cardiac and neuronal differentiation in the ESC model and these results are in concordance with previous in vivo and in vitro models.".
- ↑ "Neurobehavioral outcomes of infants exposed to MDMA (Ecstasy) and other recreational drugs during pregnancy". Neurotoxicology and Teratology 34 (3): 303–310. 2012. doi:10.1016/j.ntt.2012.02.001. PMID 22387807. Bibcode: 2012NTxT...34..303S.
- ↑ "3,4-Methylenedioxy-N-methamphetamine (ecstasy) promotes the survival of fetal dopamine neurons in culture". Neuropharmacology 55 (5): 851–859. October 2008. doi:10.1016/j.neuropharm.2008.06.062. PMID 18655796.
- ↑ 93.0 93.1 93.2 93.3 93.4 93.5 "Treatment of toxicity from amphetamines, related derivatives, and analogues: a systematic clinical review". Drug and Alcohol Dependence 150: 1–13. May 2015. doi:10.1016/j.drugalcdep.2015.01.040. PMID 25724076.
- ↑ 94.0 94.1 "Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans". British Journal of Pharmacology 166 (8): 2277–2288. August 2012. doi:10.1111/j.1476-5381.2012.01936.x. PMID 22404145.
- ↑ "Caffeine provokes adverse interactions with 3,4-methylenedioxymethamphetamine (MDMA, 'ecstasy') and related psychostimulants: mechanisms and mediators". British Journal of Pharmacology 167 (5): 946–959. November 2012. doi:10.1111/j.1476-5381.2012.02065.x. PMID 22671762.
- ↑ "Acute toxic effects of 'Ecstasy' (MDMA) and related compounds: overview of pathophysiology and clinical management". British Journal of Anaesthesia 96 (6): 678–685. June 2006. doi:10.1093/bja/ael078. PMID 16595612.
- ↑ 97.0 97.1 97.2 97.3 97.4 97.5 97.6 "Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition". Therapeutic Drug Monitoring 26 (2): 137–144. April 2004. doi:10.1097/00007691-200404000-00009. PMID 15228154. http://www.maps.org/w3pb/new/2004/2004_de_20593_2.pdf. Retrieved 25 October 2009.
- ↑ 98.0 98.1 98.2 98.3 98.4 98.5 Oxford American Handbook of Critical Care. Oxford University Press. 2008. p. 464. ISBN 978-0-19-530528-9. OCLC 1003197730.
- ↑ "3,4-methylenedioxyamfetamine (ecstasy) use reduces cognition". British Journal of Nursing (Mark Allen Publishing) 19 (2): 94–100. 2010. PMID 20235382.
- ↑ "Nephrotoxic effects of common and emerging drugs of abuse". Clinical Journal of the American Society of Nephrology 9 (11): 1996–2005. November 2014. doi:10.2215/CJN.00360114. PMID 25035273.
- ↑ "Qualitative review of serotonin syndrome, ecstasy (MDMA) and the use of other serotonergic substances: hierarchy of risk". The Australian and New Zealand Journal of Psychiatry 41 (8): 649–655. August 2007. doi:10.1080/00048670701449237. PMID 17620161.
- ↑ "MDMA interactions with pharmaceuticals and drugs of abuse". Expert Opinion on Drug Metabolism & Toxicology 16 (5): 357–369. May 2020. doi:10.1080/17425255.2020.1749262. PMID 32228243.
- ↑ 103.00 103.01 103.02 103.03 103.04 103.05 103.06 103.07 103.08 103.09 103.10 103.11 103.12 "Ecstasy (3,4-methylenedioxymethamphetamine): Cardiovascular effects and mechanisms". European Journal of Pharmacology 903. July 2021. doi:10.1016/j.ejphar.2021.174156. PMID 33971177.
- ↑ 104.0 104.1 "Interactions between bupropion and 3,4-methylenedioxymethamphetamine in healthy subjects". The Journal of Pharmacology and Experimental Therapeutics 353 (1): 102–111. April 2015. doi:10.1124/jpet.114.222356. PMID 25655950.
- ↑ "Death following ingestion of MDMA (ecstasy) and moclobemide". Addiction (Abingdon, England) 98 (3): 365–368. March 2003. doi:10.1046/j.1360-0443.2003.00292.x. PMID 12603236.
- ↑ 106.00 106.01 106.02 106.03 106.04 106.05 106.06 106.07 106.08 106.09 "Serotonin and serotonin receptors in hallucinogen action". Handbook of the Behavioral Neurobiology of Serotonin. Handbook of Behavioral Neuroscience. 31. 2020. pp. 843–863. doi:10.1016/B978-0-444-64125-0.00043-8. ISBN 978-0-444-64125-0.
- ↑ 107.0 107.1 "Poster Session II (PII 1-111): PII-41. MDMA-Induced Increases in Blood Pressure Are Not Mediated by α-Adrenergic Mechanisms and Are Not Due To Elevated Peripheral Vascular Resistance". Clinical Pharmacology & Therapeutics 91 (S1 [American Society for Clinical Pharmacology and Therapeutics Abstract of papers, 2012 Annual Meeting Gaylord National Hotel and Convention Center National Harbor, Maryland March 14–17, 2012]): S51–S93 (S66–S66). 2012. doi:10.1038/clpt.2011.361. ISSN 0009-9236. "MDMA increased heart rate (HR) by 25 bpm (p<.001), [cardiac output (CO)] by 1.75 L/min (p<0.01) but did not alter [stroke volume (SV)] or [systemic vascular resistance (SVR)]. Compared to MDMA alone the combination of MDMA + prazosin further increased HR by 24 bpm (p<0.001) and CO by 3.3L/min (p<0.02). MDMA increased systolic and diastolic blood pressure (SBP, DBP) by 26 mmHg (p<0.001 each); prazosin attenuated MDMA effects on DBP by 9.3 mmHg (p<001) but did not alter SBP. [...] MDMA increases HR, producing elevations in CO. The hypertensive effects of MDMA are not due to elevated peripheral vascular resistance and the blood pressure effects of MDMA are not attenuated by α-adrenergic blockade, suggesting that MDMA may produce CV effects through non-α-adrenergic mechanisms.".
- ↑ 108.0 108.1 108.2 108.3 108.4 108.5 "Psychological and physiological effects of MDMA ("Ecstasy") after pretreatment with the 5-HT(2) antagonist ketanserin in healthy humans". Neuropsychopharmacology 23 (4): 396–404. October 2000. doi:10.1016/S0893-133X(00)00126-3. PMID 10989266.
- ↑ 109.0 109.1 "Cardiovascular safety of psychedelic medicine: current status and future directions". Pharmacological Reports 75 (6): 1362–1380. December 2023. doi:10.1007/s43440-023-00539-4. PMID 37874530.
- ↑ "Effects of hallucinogenic drugs on the human heart". Frontiers in Pharmacology 15. 2024. doi:10.3389/fphar.2024.1334218. PMID 38370480.
- ↑ "Comparative acute effects of mescaline, lysergic acid diethylamide, and psilocybin in a randomized, double-blind, placebo-controlled cross-over study in healthy participants". Neuropsychopharmacology 48 (11): 1659–1667. October 2023. doi:10.1038/s41386-023-01607-2. PMID 37231080.
- ↑ "Update Lessons from PET Imaging Part II: A Systematic Critical Review on Therapeutic Plasma Concentrations of Antidepressants". Therapeutic Drug Monitoring 46 (2): 155–169. April 2024. doi:10.1097/FTD.0000000000001142. PMID 38287888.
- ↑ "Tools for optimising pharmacotherapy in psychiatry (therapeutic drug monitoring, molecular brain imaging and pharmacogenetic tests): focus on antidepressants". The World Journal of Biological Psychiatry 22 (8): 561–628. October 2021. doi:10.1080/15622975.2021.1878427. PMID 33977870. https://serval.unil.ch/resource/serval:BIB_6FD14CC75A02.P001/REF.pdf. Retrieved 10 April 2022.
- ↑ "Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination". The International Journal of Neuropsychopharmacology 17 (3): 371–381. March 2014. doi:10.1017/S1461145713001132. PMID 24103254. https://edoc.unibas.ch/35606/2/371.full.pdf.
- ↑ "Effects of a beta-blocker on the cardiovascular response to MDMA (Ecstasy)". Emergency Medicine Journal 27 (8): 586–589. August 2010. doi:10.1136/emj.2009.079905. PMID 20378736. https://www.zora.uzh.ch/id/eprint/40820/1/Hysek.pdf.
- ↑ "Effects of the α₂-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers". The Journal of Pharmacology and Experimental Therapeutics 340 (2): 286–294. February 2012. doi:10.1124/jpet.111.188425. PMID 22034656.
- ↑ "α₁-Adrenergic receptors contribute to the acute effects of 3,4-methylenedioxymethamphetamine in humans". Journal of Clinical Psychopharmacology 33 (5): 658–666. October 2013. doi:10.1097/JCP.0b013e3182979d32. PMID 23857311.
- ↑ "Acute psychological and physiological effects of MDMA ("Ecstasy") after haloperidol pretreatment in healthy humans". European Neuropsychopharmacology 10 (4): 289–295. July 2000. doi:10.1016/s0924-977x(00)00086-9. PMID 10871712.
- ↑ "β-Blockers, Cocaine, and the Unopposed α-Stimulation Phenomenon". Journal of Cardiovascular Pharmacology and Therapeutics 22 (3): 239–249. May 2017. doi:10.1177/1074248416681644. PMID 28399647.
- ↑ 120.0 120.1 120.2 "PDSP Database" (in zu). https://pdsp.unc.edu/databases/pdsp.php?testFreeRadio=testFreeRadio&testLigand=MDMA&kiAllRadio=all&doQuery=Submit+Query.
- ↑ 121.0 121.1 121.2 "BindingDB BDBM50010588 (RS)-3,4-(methylenedioxy)methamphetamine::1-(1,3-Benzodioxol-5-yl)-N-methyl-2-propanamine::1-(1,3-benzodioxol-5-yl)-N-methylpropan-2-amine::3,4-methylenedioxymethamphetamine::CHEMBL43048::DL-(3,4-Methylenedioxy)methamphetamine::MDMA::N,alpha-dimethyl-1,3-benzodioxole-5-ethanamine::N-Methyl-3,4-methylenedioxyamphetamine::US11767305, Compound MDMA". https://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=50010588.
- ↑ 122.0 122.1 122.2 "Psychedelics and the human receptorome". PLOS ONE 5 (2). February 2010. doi:10.1371/journal.pone.0009019. PMID 20126400. Bibcode: 2010PLoSO...5.9019R.
- ↑ 123.0 123.1 "Pharmacological characterization of designer cathinones in vitro". British Journal of Pharmacology 168 (2): 458–470. January 2013. doi:10.1111/j.1476-5381.2012.02145.x. PMID 22897747. "β-Keto-analogue cathinones also exhibited approximately 10-fold lower affinity for the TA1 receptor compared with their respective non-β-keto amphetamines. [...] Activation of TA1 receptors negatively modulates dopaminergic neurotransmission. Importantly, methamphetamine decreased DAT surface expression via a TA1 receptor-mediated mechanism and thereby reduced the presence of its own pharmacological target (Xie and Miller, 2009). MDMA and amphetamine have been shown to produce enhanced DA and 5-HT release and locomotor activity in TA1 receptor knockout mice compared with wild-type mice (Lindemann et al., 2008; Di Cara et al., 2011). Because methamphetamine and MDMA auto-inhibit their neurochemical and functional effects via TA1 receptors, low affinity for these receptors may result in stronger effects on monoamine systems by cathinones compared with the classic amphetamines.".
- ↑ "Monoamine transporter and receptor interaction profiles of a new series of designer cathinones". Neuropharmacology 79: 152–160. April 2014. doi:10.1016/j.neuropharm.2013.11.008. PMID 24275046.
- ↑ "Pharmacological profile of novel psychoactive benzofurans". British Journal of Pharmacology 172 (13): 3412–3425. July 2015. doi:10.1111/bph.13128. PMID 25765500.
- ↑ "Metabolites of the ring-substituted stimulants MDMA, methylone and MDPV differentially affect human monoaminergic systems". Journal of Psychopharmacology (Oxford, England) 33 (7): 831–841. July 2019. doi:10.1177/0269881119844185. PMID 31038382.
- ↑ "Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function". Psychopharmacology 231 (5): 875–888. March 2014. doi:10.1007/s00213-013-3303-6. PMID 24142203. PMC 3945162. https://www.researchgate.net/publication/258061356.
- ↑ 128.0 128.1 128.2 128.3 128.4 "Trace Amines and Their Receptors". Pharmacological Reviews 70 (3): 549–620. July 2018. doi:10.1124/pr.117.015305. PMID 29941461. https://www.researchgate.net/publication/325975689.
- ↑ 129.0 129.1 129.2 129.3 "In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1". The Journal of Pharmacology and Experimental Therapeutics 357 (1): 134–144. April 2016. doi:10.1124/jpet.115.229765. PMID 26791601. https://d1wqtxts1xzle7.cloudfront.net/74120533/eae6c6e62565b82d46b4d111bbea0f77b9c2-libre.pdf?1635931703=&response-content-disposition=inline%3B+filename%3DIn_Vitro_Characterization_of_Psychoactiv.pdf&Expires=1746838268&Signature=Sy4fJ90yUhxs68314NxYsW5PAaNrBGePRu35WRR4PIF-3YC7Z~sLdnCn5wfqqbLg9bDEGdt~oW55ugMP3D3jgA0BoRI~~GOb0NQOwrtfUEQK1PQs1uuN9qg5Y1ct8z5NsABm44RgtukkwRMdU6fO7OlfIsQ68hOiFk129Ll7UYqldxD2f1xhE2fTTfsxSpb8cMCJzHn7-ItqLdwnAUPFK7WggDIjmY1kCnaHLwIxMwdJCAq8L6DYzSTg7pZkbR8qlou~GXbTPQt~gYpyZTJp5hgW-7V6K5wLlQ7Z2xE7B0f9wEfuc1W1QNafg125Tr-vvAe4LEGKXV58bnn1bpfWKw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA.
- ↑ "Trace amine-associated receptors as emerging therapeutic targets". Molecular Pharmacology 76 (2): 229–235. August 2009. doi:10.1124/mol.109.055970. PMID 19389919.
- ↑ 131.0 131.1 "Entactogens: How the Name for a Novel Class of Psychoactive Agents Originated". Frontiers in Psychiatry 13. 2022. doi:10.3389/fpsyt.2022.863088. PMID 35401275.
- ↑ 132.0 132.1 132.2 132.3 132.4 132.5 132.6 132.7 "Pharmacology of Drugs Used as Stimulants". Journal of Clinical Pharmacology 61 (Suppl 2): S53–S69. August 2021. doi:10.1002/jcph.1918. PMID 34396557. "Receptor-mediated actions of amphetamine and other amphetamine derivatives [...] may involve trace amine-associated receptors (TAARs) at which amphetamine and MDMA also have significant potency.85–87 Many stimulants have potency at the rat TAAR1 in the micromolar range but tend to be about 5 to 10 times less potent at the human TAAR1, [...] Activation of the TAAR1 receptor causes inhibition of dopaminergic transmission in the mesocorticolimbic system, and TAAR1 agonists attenuated psychostimulant abuse-related behaviors.89 It is likely that TAARs contribute to the actions of specific stimulants to modulate dopaminergic, serotonergic, and glutamate signaling,90 and drugs acting on the TAAR1 may have therapeutic potential.91 In the periphery, stimulants such as MDMA and cathinone produce vasoconstriction, part of which may involve TAARs, although only relatively high concentrations produced vascular contractions resistant to a cocktail of monoamine antagonist drugs.86".
- ↑ 133.0 133.1 133.2 "Monoamine transporters and psychostimulant drugs". European Journal of Pharmacology 479 (1–3): 23–40. October 2003. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ↑ 134.0 134.1 134.2 134.3 134.4 134.5 "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry 6 (17): 1845–1859. 2006. doi:10.2174/156802606778249766. PMID 17017961.
- ↑ 135.0 135.1 "Mechanisms of neurotransmitter release by amphetamines: a review". Progress in Neurobiology 75 (6): 406–433. April 2005. doi:10.1016/j.pneurobio.2005.04.003. PMID 15955613.
- ↑ 136.0 136.1 "Molecular Mechanisms of Amphetamines". Substance Use Disorders. Handbook of Experimental Pharmacology. 258. 2020. pp. 265–297. doi:10.1007/164_2019_251. ISBN 978-3-030-33678-3.
- ↑ 137.0 137.1 "Post-translational mechanisms in psychostimulant-induced neurotransmitter efflux". Pharmacological Advances in Central Nervous System Stimulants. Advances in Pharmacology. 99. 2024. pp. 1–33. doi:10.1016/bs.apha.2023.10.003. ISBN 978-0-443-21933-7. https://books.google.com/books?id=2Sr6EAAAQBAJ&pg=PA1.
- ↑ 138.0 138.1 "The polypharmacology of psychedelics reveals multiple targets for potential therapeutics". Neuron 113 (19): 3129–3142.e9. July 2025. doi:10.1016/j.neuron.2025.06.012. PMID 40683247. https://www.cell.com/cms/10.1016/j.neuron.2025.06.012/attachment/7d8365fe-51f3-4a28-bf40-9999bec837f6/mmc11.pdf.
- ↑ 139.0 139.1 139.2 139.3 139.4 139.5 139.6 139.7 139.8 "(±)-MDMA and its enantiomers: potential therapeutic advantages of R(-)-MDMA". Psychopharmacology 235 (2): 377–392. February 2018. doi:10.1007/s00213-017-4812-5. PMID 29248945.
- ↑ 140.0 140.1 140.2 140.3 140.4 140.5 140.6 "3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro". Molecular Pharmacology 63 (6): 1223–1229. June 2003. doi:10.1124/mol.63.6.1223. PMID 12761331.
- ↑ 141.0 141.1 141.2 141.3 141.4 "Effect of the R(-) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors". Neuroscience Letters 177 (1–2): 111–115. August 1994. doi:10.1016/0304-3940(94)90057-4. PMID 7824160.
- ↑ "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorganic & Medicinal Chemistry 19 (23): 7044–7048. December 2011. doi:10.1016/j.bmc.2011.10.007. PMID 22037049.
- ↑ "Neuronal Functions and Emerging Pharmacology of TAAR1". Taste and Smell. Topics in Medicinal Chemistry. 23. Cham: Springer International Publishing. 2014. pp. 175–194. doi:10.1007/7355_2014_78. ISBN 978-3-319-48925-4. "Interestingly, the concentrations of amphetamine found to be necessary to activate TAAR1 are in line with what was found in drug abusers [3, 51, 52]. Thus, it is likely that some of the effects produced by amphetamines could be mediated by TAAR1. Indeed, in a study in mice, MDMA effects were found to be mediated in part by TAAR1, in a sense that MDMA auto-inhibits its neurochemical and functional actions [46]. Based on this and other studies (see other section), it has been suggested that TAAR1 could play a role in reward mechanisms and that amphetamine activity on TAAR1 counteracts their known behavioral and neurochemical effects mediated via dopamine neurotransmission."
- ↑ "A narrative review of the neuropharmacology of synthetic cathinones-Popular alternatives to classical drugs of abuse". Human Psychopharmacology 38 (3). May 2023. doi:10.1002/hup.2866. PMID 36866677. "Another feature that distinguishes [synthetic cathinones (SCs)] from amphetamines is their negligible interaction with the trace amine associated receptor 1 (TAAR1). Activation of this receptor reduces the activity of dopaminergic neurones, thereby reducing psychostimulatory effects and addictive potential (Miller, 2011; Simmler et al., 2016). Amphetamines are potent agonists of this receptor, making them likely to self‐inhibit their stimulating effects. In contrast, SCs show negligible activity towards TAAR1 (Kolaczynska et al., 2021; Rickli et al., 2015; Simmler et al., 2014, 2016). [...] It is worth noting, however, that for TAAR1 there is considerable species variability in its interaction with ligands, and it is possible that the in vitro activity of [rodent TAAR1 agonists] may not translate into activity in the human body (Simmler et al., 2016). The lack of self‐regulation by TAAR1 may partly explain the higher addictive potential of SCs compared to amphetamines (Miller, 2011; Simmler et al., 2013).".
- ↑ "Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA)". The Journal of Neuroscience 31 (47): 16928–16940. November 2011. doi:10.1523/JNEUROSCI.2502-11.2011. PMID 22114263.
- ↑ "MDMA enhances empathy-like behaviors in mice via 5-HT release in the nucleus accumbens". Science Advances 10 (17). April 2024. doi:10.1126/sciadv.adl6554. PMID 38657057. Bibcode: 2024SciA...10L6554R.
- ↑ 147.0 147.1 147.2 147.3 147.4 147.5 147.6 147.7 "Beyond ecstasy: Alternative entactogens to 3,4-methylenedioxymethamphetamine with potential applications in psychotherapy". Journal of Psychopharmacology (Oxford, England) 35 (5): 512–536. May 2021. doi:10.1177/0269881120920420. PMID 32909493.
- ↑ "Role of 5-HT1A receptors in the basolateral amygdala on 3,4-methylenedioxymethamphetamine-induced prosocial effects in mice". European Journal of Pharmacology 946. May 2023. doi:10.1016/j.ejphar.2023.175653. PMID 36907260.
- ↑ "Distinct neural mechanisms for the prosocial and rewarding properties of MDMA". Science Translational Medicine 11 (522). December 2019. doi:10.1126/scitranslmed.aaw6435. PMID 31826983.
- ↑ "3,4-Methylenedioxymethamphetamine Increases Affiliative Behaviors in Squirrel Monkeys in a Serotonin 2A Receptor-Dependent Manner". Neuropsychopharmacology 42 (10): 1962–1971. September 2017. doi:10.1038/npp.2017.80. PMID 28425496.
- ↑ "Effect of drugs of abuse on social behaviour: a review of animal models". Behavioural Pharmacology 26 (6): 541–570. September 2015. doi:10.1097/FBP.0000000000000162. PMID 26221831.
- ↑ "Therapeutic mechanisms of psychedelics and entactogens". Neuropsychopharmacology 49 (1): 104–118. January 2024. doi:10.1038/s41386-023-01666-5. PMID 37488282.
- ↑ 153.0 153.1 153.2 153.3 "Balancing Therapeutic Efficacy and Safety of MDMA and Novel MDXX Analogues as Novel Treatments for Autism Spectrum Disorder". Psychedelic Medicine (New Rochelle, N.Y.) 1 (3): 166–185. 2023. doi:10.1089/psymed.2023.0023. PMID 40046567. "It is postulated that MDMA-induced neuronal apoptosis arises from directly stimulating the 5HT2A receptor. However, it is unclear whether MDMA binds here directly or whether one of its active metabolites (for example, MDA exhibits a 5-HT2A affinity almost 10-fold better than MDMA) is responsible.70,80,81 In addition, R-MDMA more potently activates 5-HT2A second messenger signaling, with S-MDMA having a minimal effect and racemic MDMA acting as a weak partial agonist.".
- ↑ "3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions". The Journal of Pharmacology and Experimental Therapeutics 297 (3): 846–852. June 2001. doi:10.1016/S0022-3565(24)29607-5. PMID 11356903. https://bibliography.maps.org/resources/download/983.
- ↑ "Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain". Pharmacology, Biochemistry, and Behavior 90 (2): 208–217. August 2008. doi:10.1016/j.pbb.2008.02.018. PMID 18403002. "Determining the role of specific 5-HT receptors in MDMA's effects in vivo is complicated by the presence of 14 different 5-HT receptor subtypes (Barnes and Sharp, 1999; Hoyer et al., 2002), many of which affect DA function (Alex and Pehek, 2007; Bubar and Cunningham, 2006). Nonetheless, there is general agreement that 5-HT1B, 5-HT2A and 5-HT2C receptor subtypes influence locomotor effects of MDMA. Hyperactivity produced by MDMA is mimicked by administration of the 5-HT1B agonist RU-24969 (Rempel et al., 1993), and 5-HT1B antagonists inhibit MDMA-induced ambulation (Callaway and Geyer, 1992b; Fletcher et al., 2002; McCreary et al., 1999). 5-HT2A antagonists reduce ambulation produced by MDMA (Fletcher et al., 2002; Kehne et al., 1996), whereas 5-HT2C antagonists markedly enhance it (Bankson and Cunningham, 2002; Fletcher et al., 2006). Taken together, these data reveal that 5-HT1B and 5-HT2A receptors facilitate, while 5-HT2C receptors suppress, forward locomotion evoked by MDMA administration. Importantly, the neural circuits underlying serotonergic modulation of MDMA-induced activity are largely unexplored.".
- ↑ "Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors". Neuropsychopharmacology 26 (1): 40–52. January 2002. doi:10.1016/S0893-133X(01)00345-1. PMID 11751031.
- ↑ "The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA)". Neuroscience and Biobehavioral Reviews 27 (3): 199–217. May 2003. doi:10.1016/s0149-7634(03)00031-9. PMID 12788333. "[...] 5-HT1B receptor agonists elicit locomotor hyperactivity similar to MDMA [247] and transgenic mice lacking the SERT or 5-HT1B receptor show no or reduced MDMA-induced locomotion and a behavioural pattern qualitatively reminiscent of other amphetamines [262,27]. Prior 5-HT depletion or pre-treatment of animals with SSRIs or 5-HT1B receptor antagonists block low dose MDMA hyperkinesis [42,44]. Activation of this receptor subtype may be fundamental in defining MDMAspecific locomotor activity. The 5-HT2A antagonist MDL 100,907 blocked high dose (20 mg/kg) but not low dose (3 mg/kg) MDMA-induced locomotion [157].5-HT2C receptor antagonists potentiated locomotor activation after low dose MDMA [20,115]. It has been proposed that unmasking of 5-HT1B receptor-mediated hyperactivity via 5-HT2C antagonism is only possible in the presence of the elevated DA and 5-HT concentrations seen after MDMA [19].".
- ↑ "5-HT2C receptors in the nucleus accumbens constrain the rewarding effects of MDMA". Molecular Psychiatry 30 (11): 5405–5416. July 2025. doi:10.1038/s41380-025-03128-4. PMID 40707786.
- ↑ "The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens". Journal of Psychopharmacology (Oxford, England) 31 (1): 127–143. January 2017. doi:10.1177/0269881116677104. PMID 27903793.
- ↑ "Behavioral Psychopharmacology of MDMA and MDMA-Like Drugs: A Review of Human and Animal Studies". Addiction Research & Theory (Informa UK Limited) 10 (1): 43–67. 1 January 2002. doi:10.1080/16066350290001704. ISSN 1606-6359.
- ↑ "Mice in ecstasy: advanced animal models in the study of MDMA". Current Pharmaceutical Biotechnology 11 (5): 421–433. August 2010. doi:10.2174/138920110791591508. PMID 20420576.
- ↑ "Serotonin 5-HT2B receptors are required for 3,4-methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro". The Journal of Neuroscience 28 (11): 2933–2940. March 2008. doi:10.1523/JNEUROSCI.5723-07.2008. PMID 18337424. "Here we show that acute pharmacological inhibition or genetic ablation of the 5-HT2B receptor in mice completely abolishes MDMA-induced hyperlocomotion and 5-HT release in nucleus accumbens and ventral tegmental area. Furthermore, the 5-HT2B receptor dependence of MDMA-stimulated release of endogenous 5-HT from superfused midbrain synaptosomes suggests that 5-HT2B receptors act, unlike any other 5-HT receptor, presynaptically to affect MDMA-stimulated 5-HT release.".
- ↑ "Role of Serotonin via 5-HT2B Receptors in the Reinforcing Effects of MDMA in Mice". PLOS ONE 4 (11). 23 November 2009. doi:10.1371/journal.pone.0007952. ISSN 1932-6203. PMID 19956756. Bibcode: 2009PLoSO...4.7952D.
- ↑ "Positive regulation of raphe serotonin neurons by serotonin 2B receptors". Neuropsychopharmacology 43 (7): 1623–1632. 2018. doi:10.1038/s41386-018-0013-0. ISSN 0893-133X. PMID 29453444.
- ↑ 165.0 165.1 "Beyond Ecstasy: Progress in Developing and Understanding a Novel Class of Therapeutic Medicine". PS2023 [Psychedelic Science 2023, June 19–23, 2023, Denver, Colorado]. Denver, CO: Multidisciplinary Association for Psychedelic Studies. 23 June 2023. https://2023.psychedelicscience.org/sessions/beyond-ecstasy-progress-in-developing-and-understanding-a-novel-class-of-therapeutic-medicine/.
- ↑ 166.0 166.1 "Better Than Ecstasy: Progress in Developing a Novel Class of Therapeutic with Matthew Baggott, PhD.". 6 March 2024. https://www.youtube.com/watch?v=OnhJvKxwfZI&t=1048.
- ↑ 167.0 167.1 "Therapeutic and adverse actions of serotonin transporter substrates". Pharmacology & Therapeutics 95 (1): 73–88. July 2002. doi:10.1016/s0163-7258(02)00234-6. PMID 12163129.
- ↑ "Designer drugs: mechanism of action and adverse effects". Archives of Toxicology 94 (4): 1085–1133. April 2020. doi:10.1007/s00204-020-02693-7. PMID 32249347. PMC 7225206. Bibcode: 2020ArTox..94.1085L. https://repositorium.meduniwien.ac.at/obvumwoa/content/titleinfo/5270457/full.pdf.
- ↑ 169.0 169.1 169.2 169.3 169.4 169.5 169.6 "Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies". Human Psychopharmacology 16 (8): 589–598. December 2001. doi:10.1002/hup.348. PMID 12404538.
- ↑ 170.0 170.1 "Methylenedioxymethamphetamine (MDMA) in Psychiatry: Pros, Cons, and Suggestions". Journal of Clinical Psychopharmacology 38 (6): 632–638. December 2018. doi:10.1097/JCP.0000000000000962. PMID 30303861.
- ↑ 171.0 171.1 171.2 171.3 171.4 "MDMA-Induced Dissociative State not Mediated by the 5-HT2A Receptor". Frontiers in Pharmacology 8. 2017. doi:10.3389/fphar.2017.00455. PMID 28744219.
- ↑ 172.0 172.1 172.2 "3,4-methylenedioxymethamphetamine (MDMA): current perspectives". Substance Abuse and Rehabilitation 4: 83–99. 2013. doi:10.2147/SAR.S37258. PMID 24648791.
- ↑ 173.0 173.1 "Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") are attenuated by the serotonin uptake inhibitor citalopram". Neuropsychopharmacology 22 (5): 513–521. May 2000. doi:10.1016/S0893-133X(99)00148-7. PMID 10731626.
- ↑ 174.0 174.1 174.2 174.3 "Pharmacology of MDMA- and Amphetamine-Like New Psychoactive Substances". New Psychoactive Substances. Handbook of Experimental Pharmacology. 252. 2018. pp. 143–164. doi:10.1007/164_2018_113. ISBN 978-3-030-10560-0. "MDMA is also a low-potency partial agonist of the 5-HT2A receptor. Although not frequent, mild hallucinogen-like effects of MDMA have been reported, which may be attributable to 5-HT2A agonism (Nichols 2004; Liechti et al. 2000). MDA, the active metabolite of MDMA (Hysek et al. 2011), shows a tenfold higher potency for 5-HT2A agonism than MDMA (Rickli et al. 2015c). MDA likely contributes to the mode of action of MDMA and might contribute to the mild hallucinogenic effects of MDMA."
- ↑ 175.0 175.1 175.2 175.3 175.4 175.5 175.6 175.7 Cite error: Invalid
<ref>tag; no text was provided for refs namedStraumann_2024 - ↑ Chemistry and Structure-Activity Relationships of Psychedelics. Current Topics in Behavioral Neurosciences. 36. 2018. pp. 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2. https://bitnest.netfirms.com/external/10.1007/7854_2017_475. "Although the most active tryptamine hallucinogens are N,N-dialkylated, the phenethylamines generally cannot tolerate even a single N-substitution. Even small groups such as methyl or ethyl (see Table 2) abolish their hallucinogenic activity."
- ↑ "Structure-Activity Relationships of the Classic Hallucinogens and Their Analogs". Hallucinogens: An Update. National Institute on Drug Abuse Research Monograph Series. 146. National Institute on Drug Abuse. 1994. pp. 74–91. https://bibliography.maps.org/resources/download/11534. "[MDA] is also remarkable because the N-methyl homolog 3,4 (MDMA) has biological activity, although the nature of its action places it outside of this review. No other phenethylamine hallucinogen retains central activity on N-methylation."
- ↑ "Effect of Hallucinogens on Unconditioned Behavior". Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. 36. 2018. pp. 159–199. doi:10.1007/7854_2016_466. ISBN 978-3-662-55878-2. "[MDxx] have been assessed in head twitch studies. Racemic [MDA] and S-(+)-MDA reportedly induce WDS in monkeys and rats, respectively (Schlemmer and Davis 1986; Hiramatsu et al. 1989). Although [MDMA] does not induce the HTR in mice, both of the stereoisomers of MDMA have been shown to elicit the response (Fantegrossi et al. 2004, 2005b). 5-HT depletion inhibits the response to S-(+)-MDMA but does not alter the response to R-(−)-MDMA, suggesting the isomers act through different mechanisms (Fantegrossi et al. 2005b). This suggestion is consistent with the fact that S-(+)- and R-(−)-MDMA exhibit qualitatively distinct pharmacological profiles, with the S-(+)isomer working primarily as a monoamine releaser (Johnson et al. 1986; Baumann et al. 2008; Murnane et al. 2010) and the R-(−)-enantiomer acting directly through 5-HT2A receptors (Lyon et al. 1986; Nash et al. 1994). In contrast to their effects in mice, Hiramatsu reported that S-(+)- and R-(−)-MDMA fail to produce WDS in rats (Hiramatsu et al. 1989). The discrepant findings with MDMA in mice and rats may reflect species differences in sensitivity to the HTR (see below for further discussion)."
- ↑ Dunlap LE (2022). Development of Non-Hallucinogenic Psychoplastogens (Thesis). University of California, Davis. Retrieved 18 November 2024.
Finally, since R-MDMA is known to partially substitute for LSD in animal models we decided to test both compounds in the head twitch response assay (HTR) (FIG 3.3C).3 The HTR is a well-validated mouse model for predicting the hallucinogenic potential of test drugs. Serotonergic psychedelics will cause a rapid back and forth head movement in mice. The potency measured in the HTR assay has been shown to correlate very well with the human potencies of psychedelics.18 Neither R-MDMA or [...] produced any head twitches at all doses tested, suggesting that neither has high hallucinogenic potential.
- ↑ "Psychedelics" (in en). Pharmacological Reviews 68 (2): 264–355. 2016. doi:10.1124/pr.115.011478. ISSN 0031-6997. PMID 26841800.
- ↑ "Structure-activity relationships of MDMA-like substances". NIDA Research Monograph 94: 1–29. 1989. PMID 2575223.
- ↑ "Structure-activity relationships of MDMA and related compounds: a new class of psychoactive drugs?". Annals of the New York Academy of Sciences 600: 613–23; discussion 623–5. 1990. doi:10.1007/978-1-4613-1485-1_7. PMID 1979214.
- ↑ "Serotonin 5-HT2B receptor agonism and valvular heart disease: implications for the development of psilocybin and related agents". Expert Opinion on Drug Safety 22 (10): 881–883. 2023. doi:10.1080/14740338.2023.2248883. PMID 37581427.
- ↑ 184.0 184.1 184.2 "The risk of chronic psychedelic and MDMA microdosing for valvular heart disease". Journal of Psychopharmacology (Oxford, England) 37 (9): 876–890. September 2023. doi:10.1177/02698811231190865. PMID 37572027. https://unlimitedsciences.org/wp-content/uploads/2024/01/tagen-et-al-2023-the-risk-of-chronic-psychedelic-and-mdma-microdosing-for-valvular-heart-disease.pdf. "[...] Both [MDMA and MDA] bind to the human 5-HT2B receptor, although with a 5-fold lower Ki value for MDA compared to MDMA (Ray, 2010; Setola et al., 2003). Both compounds were agonists in an assay of PI hydrolysis, with MDA (EC50=190nM) 10-fold more potent than MDMA (EC50=2000 nM) in addition to greater intrinsic efficacy (90% vs 32%) (Setola et al., 2003). [...] A 50mg dose of MDMA resulted in a mean plasma Cmax 266nM for MDMA and 28.5nM for MDA (de la Torre et al., 2000).".
- ↑ "Serotonergic drugs and valvular heart disease". Expert Opinion on Drug Safety 8 (3): 317–329. May 2009. doi:10.1517/14740330902931524. PMID 19505264.
- ↑ "Serotonin releasing agents. Neurochemical, therapeutic and adverse effects". Pharmacology, Biochemistry, and Behavior 71 (4): 825–836. April 2002. doi:10.1016/s0091-3057(01)00669-4. PMID 11888573.
- ↑ 187.0 187.1 "Neurotoxicity of MDMA: Main effects and mechanisms". Experimental Neurology 347. January 2022. doi:10.1016/j.expneurol.2021.113894. PMID 34655576. https://www.didyouno.fr/wp-content/uploads/2023/03/1-s2.0-S0014488621003022-main.pdf.
- ↑ 188.0 188.1 "An integrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine". Neurotoxicology 19 (3): 427–441. June 1998. PMID 9621349. https://www.researchgate.net/publication/13663847.
- ↑ "The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy")". Pharmacol Rev 55 (3): 463–508. September 2003. doi:10.1124/pr.55.3.3. PMID 12869661.
- ↑ "Potentiation of 3,4-methylenedioxymethamphetamine-induced dopamine release and serotonin neurotoxicity by 5-HT2 receptor agonists". Eur J Pharmacol 264 (3): 325–330. November 1994. doi:10.1016/0014-2999(94)90669-6. PMID 7698172.
- ↑ "Methylenedioxymethamphetamine-induced hyperthermia and neurotoxicity are independently mediated by 5-HT2 receptors". Brain Res 529 (1–2): 85–90. October 1990. doi:10.1016/0006-8993(90)90813-q. PMID 1980848.
- ↑ "Potentiation of (DL)-3,4-methylenedioxymethamphetamine (MDMA)-induced toxicity by the serotonin 2A receptior partial agonist d-lysergic acid diethylamide (LSD), and the protection of same by the serotonin 2A/2C receptor antagonist MDL 11,939". Neuroscience Research Communications 35 (2): 83–95. 2004. doi:10.1002/nrc.20023.
- ↑ "Absolute configuration and psychotomimetic activity". NIDA Research Monograph (22): 8–15. 1978. PMID 101890. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=2ab674b010611df18c029a78f6d17e52dba5f82f.
- ↑ "Differential effects of intravenous R,S-(+/-)-3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and its S(+)- and R(-)-enantiomers on dopamine transmission and extracellular signal regulated kinase phosphorylation (pERK) in the rat nucleus accumbens shell and core". Journal of Neurochemistry 102 (1): 121–132. July 2007. doi:10.1111/j.1471-4159.2007.04451.x. PMID 17564678.
- ↑ "Separating the agony from ecstasy: R(-)-3,4-methylenedioxymethamphetamine has prosocial and therapeutic-like effects without signs of neurotoxicity in mice". Neuropharmacology 128: 196–206. January 2018. doi:10.1016/j.neuropharm.2017.10.003. PMID 28993129.
- ↑ 196.0 196.1 "Is the stereoisomer R-MDMA a safer version of MDMA?". Neuropsychopharmacology 50 (2): 360–361. October 2024. doi:10.1038/s41386-024-02009-8. PMID 39448866.
- ↑ "Drugs of Abuse Affecting 5-HT2B Receptors". 5-HT2B Receptors. The Receptors. 35. Cham: Springer International Publishing. 2021. pp. 277–289. doi:10.1007/978-3-030-55920-5_16. ISBN 978-3-030-55919-9. "Notably, in a study by Rickli and colleagues, MDMA did not activate the 5-HT2B receptor in the functional assay at investigated concentrations (EC50 > 20 μM); however, [MDA], the main psychoactive N-demethylated phase I metabolite of MDMA, potently activated the receptor at submicromolar concentrations [14]. This suggests that the metabolite MDA rather than MDMA itself may lead to valvulopathy and that there could be a significant metabolic contribution to MDMA-induced effects and adverse effect."
- ↑ "Mephedrone and MDMA: A comparative review". Brain Research 1735. May 2020. doi:10.1016/j.brainres.2020.146740. PMID 32087112. "A controlled study on eight experienced MDMA users reported that 1.5 mg/kg (comparable to what was deemed a typical dosage amount) consumed orally resulted in the subjective effects peaking within 2 h of ingestion (Harris et al., 2002). Other research indicates effects to emerge between 20 and 60 min, with them peaking between 60 and 90 min and lasting up to 5 h (Green et al., 2003). A dose of 100 mg has a half-life of 8–9h(De la Torre et al., 2004), although as mentioned above, users are unaware of the dose they ingest.".
- ↑ "Lisdexamfetamine Dimesylate: Prodrug Delivery, Amphetamine Exposure and Duration of Efficacy". Clinical Drug Investigation 36 (5): 341–356. May 2016. doi:10.1007/s40261-015-0354-y. PMID 27021968.
- ↑ "A review of the clinical pharmacology of methamphetamine". Addiction (Abingdon, England) 104 (7): 1085–1099. July 2009. doi:10.1111/j.1360-0443.2009.02564.x. PMID 19426289. "Metabolism does not appear to be altered by chronic exposure, thus dose escalation appears to arise from pharmacodynamic rather than pharmacokinetic tolerance [24]. [...] The terminal plasma half-life of methamphetamine of approximately 10 hours is similar across administration routes, but with substantial inter-individual variability. Acute effects persist for up to 8 hours following a single moderate dose of 30 mg [30]. [...] peak plasma methamphetamine concentration occurs after 4 hours [35]. Nevertheless, peak cardiovascular and subjective effects occur rapidly (within 5–15 minutes). The dissociation between peak plasma concentration and clinical effects indicates acute tolerance, which may reflect rapid molecular processes such as redistribution of vesicular monoamines and internalization of monoamine receptors and transporters [6,36]. Acute subjective effects diminish over 4 hours, while cardiovascular effects tend to remain elevated. This is important, as the marked acute tachyphylaxis to subjective effects may drive repeated use within intervals of 4 hours, while cardiovascular risks may increase [11,35].".
- ↑ "A review of amphetamine extended release once-daily options for the management of attention-deficit hyperactivity disorder". Expert Review of Neurotherapeutics 24 (4): 421–432. April 2024. doi:10.1080/14737175.2024.2321921. PMID 38391788. "For several decades, clinical benefits of amphetamines have been limited by the pharmacologic half-life of around 4 hours. Although higher doses can produce higher maximum concentrations, they do not affect the half-life of the dose. Therefore, to achieve longer durations of effect, stimulants had to be dosed at least twice daily. Further, these immediate-release doses were found to have their greatest effect shortly after administration, with a rapid decline in effect after reaching peak blood concentrations. The clinical correlation of this was found in comparing math problems attempted and solved between a mixed amphetamine salts preparation (MAS) 10 mg once at 8 am vs 8 am followed by 12 pm [14]. The study also demonstrated the phenomenon of acute tolerance, where even if blood concentrations were maintained over the course of the day, clinical efficacy in the form of math problems attempted and solved would diminish over the course of the day. These findings eventually led to the development of a once daily preparation (MAS XR) [15], which is a composition of 50% immediate-release beads and 50% delayed release beads intended to mimic this twice-daily dosing with only a single administration.".
- ↑ "Development of a Semimechanistic Pharmacokinetic-Pharmacodynamic Model Describing Dextroamphetamine Exposure and Striatal Dopamine Response in Rats and Nonhuman Primates following a Single Dose of Dextroamphetamine". The Journal of Pharmacology and Experimental Therapeutics 369 (1): 107–120. April 2019. doi:10.1124/jpet.118.254508. PMID 30733244. "Acute tolerance has been demonstrated for methamphetamine in rats (Segal and Kuczenski, 2006), and for D-amphetamine in rats (Lewander, 1971), [non-human primates (NHPs)] (Jedema et al., 2014) and humans (Angrist et al., 1987; Brauer et al., 1996; Dolder et al., 2017). In vivo measurement of dopamine by microdialysis was used in rats and NHPs to evaluate these time-dependent effects. In humans, various subjective measures of mood related to the drug's euphoric effects were observed to decline more rapidly than plasma concentrations following D-amphetamine oral doses ranging from 20 to 40 mg (Angrist et al., 1987; Brauer et al., 1996; Dolder et al., 2017). Whereas peak plasma concentrations and subjective effects occurred between 2 and 4 hours following administration, drug effect measures had largely returned to baseline values by 8 hours despite continued exposure to the drug (mean half-life = 8 hours following a 40 mg dose (Dolder et al., 2017)).".
- ↑ "Methamphetamine and MDMA Neurotoxicity: Biochemical and Molecular Mechanisms". Handbook of Neurotoxicity. Cham: Springer International Publishing. 2021. pp. 1–24. doi:10.1007/978-3-030-71519-9_80-1. ISBN 978-3-030-71519-9. "Injections of large doses of MDMA cause massive release of 5-HT from presynaptic vesicles, followed by a rapid decrease in 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) levels and decreased TPH activity (Górska et al., 2018; Lyles & Cadet, 2003). There do not appear to be losses of 5-HT uptake sites at early time points after MDMA administration (Lyles & Cadet, 2003). [...] MDMA also perturbs the function of SERT (Green et al., 2003), a marker of the integrity of serotonin neurons (Blakely et al., 1994). By virtue of its moderating synaptic 5-HT levels, SERT is crucial for the process of 5-HT neurotransmission (Green et al., 2003). MDMA downregulates SERT function without altering SERT mRNA or protein expression, and this rapid downregulation is sustained for at least 90 min and is dose-dependent (Kivell et al., 2010)."
- ↑ "MDMA causes a redistribution of serotonin transporter from the cell surface to the intracellular compartment by a mechanism independent of phospho-p38-mitogen activated protein kinase activation". Neuroscience 168 (1): 82–95. June 2010. doi:10.1016/j.neuroscience.2010.03.018. PMID 20298763.
- ↑ "MDMA regulates serotonin transporter function via a Protein kinase C dependent mechanism". Journal of Addiction & Prevention 1 (1): 5. April 2013. ISSN 2330-2178. https://www.researchgate.net/publication/256328051.
- ↑ 206.0 206.1 "MDMA and TAAR1-mediated RhoA Activation in Serotonin Neurons". The FASEB Journal 34 (S1): 1. 2020. doi:10.1096/fasebj.2020.34.s1.05856. ISSN 0892-6638.
- ↑ 207.0 207.1 "3,4-methylenedioxymethamphetamine (MDMA) stimulates activation of TAAR1 and subsequent neurotransmitter transporter internalization in serotonin neurons". The FASEB Journal 36 (S1). 2022. doi:10.1096/fasebj.2022.36.S1.R5394. ISSN 0892-6638.
- ↑ "Antagonists and substrates differentially regulate serotonin transporter cell surface expression in serotonergic neurons". European Journal of Pharmacology 629 (1–3): 63–67. March 2010. doi:10.1016/j.ejphar.2009.12.010. PMID 20006597. "Our results show that exposure to the SSRIs citalopram, fluoxetine, sertraline and paroxetine all induced SERT internalization, but with different efficacies. The substrates 5-HT and MDMA also induced SERT internalization, while cocaine elevated SERT cell surface expression.".
- ↑ "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse (New York, N.Y.) 39 (1): 32–41. January 2001. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ↑ "Studies of the biogenic amine transporters. 14. Identification of low-efficacy "partial" substrates for the biogenic amine transporters". The Journal of Pharmacology and Experimental Therapeutics 341 (1): 251–262. April 2012. doi:10.1124/jpet.111.188946. PMID 22271821.
- ↑ "The new psychoactive substances 5-(2-aminopropyl)indole (5-IT) and 6-(2-aminopropyl)indole (6-IT) interact with monoamine transporters in brain tissue". Neuropharmacology 101: 68–75. February 2016. doi:10.1016/j.neuropharm.2015.09.004. PMID 26362361.
- ↑ "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology 559 (2–3): 132–137. March 2007. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ↑ "2-Aminoindan and its ring-substituted derivatives interact with plasma membrane monoamine transporters and α2-adrenergic receptors". Psychopharmacology 236 (3): 989–999. March 2019. doi:10.1007/s00213-019-05207-1. PMID 30904940.
- ↑ "Dopamine-releasing agents". Dopamine Transporters: Chemistry, Biology and Pharmacology. Hoboken [NJ]: Wiley. July 2008. pp. 305–320. ISBN 978-0-470-11790-3. OCLC 181862653. https://bitnest.netfirms.com/external/Books/Dopamine-releasing-agents_c11.pdf.
- ↑ "Pharmacological characterization of 3,4-methylenedioxyamphetamine (MDA) analogs and two amphetamine-based compounds: N,α-DEPEA and DPIA". European Neuropsychopharmacology 59: 9–22. June 2022. doi:10.1016/j.euroneuro.2022.03.006. PMID 35378384. https://www.researchgate.net/publication/359686098.
- ↑ "Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans". The Journal of Pharmacology and Experimental Therapeutics 290 (1): 136–145. July 1999. doi:10.1016/S0022-3565(24)34877-3. PMID 10381769.
- ↑ 217.0 217.1 "Non-linear pharmacokinetics of MDMA ('ecstasy') in humans". British Journal of Clinical Pharmacology 49 (2): 104–109. February 2000. doi:10.1046/j.1365-2125.2000.00121.x. PMID 10671903.
- ↑ "Pharmacology of MDMA in humans". Annals of the New York Academy of Sciences 914 (1): 225–237. September 2000. doi:10.1111/j.1749-6632.2000.tb05199.x. PMID 11085324. Bibcode: 2000NYASA.914..225D.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedDrugBank - ↑ 220.0 220.1 "Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults". Therapeutic Drug Monitoring 30 (3): 320–332. June 2008. doi:10.1097/FTD.0b013e3181684fa0. PMID 18520604.
- ↑ "Direct determination of glucuronide and sulfate of 4-hydroxy-3-methoxymethamphetamine, the main metabolite of MDMA, in human urine". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 857 (1): 123–129. September 2007. doi:10.1016/j.jchromb.2007.07.003. PMID 17643356.
- ↑ "Stereospecific analysis and enantiomeric disposition of 3, 4-methylenedioxymethamphetamine (Ecstasy) in humans". Clinical Chemistry 45 (7): 1058–1069. July 1999. doi:10.1093/clinchem/45.7.1058. PMID 10388483.
- ↑ "Nonlinear pharmacokinetics of (+/-)3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses". The Journal of Pharmacology and Experimental Therapeutics 327 (1): 38–44. October 2008. doi:10.1124/jpet.108.141366. PMID 18591215.
- ↑ "Electrochemical and spectroscopic characterisation of amphetamine-like drugs: application to the screening of 3,4-methylenedioxymethamphetamine (MDMA) and its synthetic precursors". Analytica Chimica Acta 596 (2): 231–241. July 2007. doi:10.1016/j.aca.2007.06.027. PMID 17631101. Bibcode: 2007AcAC..596..231M.
- ↑ "Synthesis and cytotoxic profile of 3,4-methylenedioxymethamphetamine ("ecstasy") and its metabolites on undifferentiated PC12 cells: A putative structure-toxicity relationship". Chemical Research in Toxicology 19 (10): 1294–1304. October 2006. doi:10.1021/tx060123i. PMID 17040098. https://estudogeral.sib.uc.pt/bitstream/10316/12872/1/Synthesis%20and%20Cytotoxic%20Profile.pdf. Retrieved 24 September 2019.
- ↑ "Reductive aminations of carbonyl compounds with borohydride and borane reducing agents.". Organic Reactions (Hoboken, New Jersey, United States) 59: 59. April 2004. doi:10.1002/0471264180.or059.01. ISBN 978-0-471-26418-7.
- ↑ "A study of impurities in intermediates and 3,4-methylenedioxymethamphetamine (MDMA) samples produced via reductive amination routes". Forensic Science International 155 (2–3): 141–157. December 2005. doi:10.1016/j.forsciint.2004.11.013. PMID 16226151.
- ↑ "Impurity profiling of seized MDMA tablets by capillary gas chromatography". Analytical and Bioanalytical Chemistry 374 (2): 274–281. September 2002. doi:10.1007/s00216-002-1477-6. PMID 12324849.
- ↑ "A study of the precursors, intermediates and reaction by-products in the synthesis of 3,4-methylenedioxymethylamphetamine and its application to forensic drug analysis". Forensic Science International 60 (3): 189–202. August 1993. doi:10.1016/0379-0738(93)90238-6. PMID 7901132.
- ↑ World Drug Report 2014. Vienna, Austria: United Nations Office on Drugs and Crime. June 2014. pp. 2, 3, 123–152. ISBN 978-92-1-056752-7. http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Retrieved 1 December 2014.
- ↑ "Early Warning - MDMA and MDA Producers Using Ocotea Cymbarum as a Precursor". DEA Microgram Newsletter (Drug Enforcement Agency, U.S. Department of Justice) 38 (11): 166. 11 November 2005. https://www.justice.gov/dea/pr/micrograms/2005/mg1105.pdf.
- ↑ "Disposition of MDMA and metabolites in human sweat following controlled MDMA administration". Clinical Chemistry 55 (3): 454–462. March 2009. doi:10.1373/clinchem.2008.117093. PMID 19168553.
- ↑ Disposition of toxic drugs and chemicals in man (9th ed.). Seal Beach, Ca.: Biomedical Publications. 2011. pp. 1078–1080. ISBN 978-0-9626523-8-7.
- ↑ 234.0 234.1 234.2 234.3 234.4 234.5 234.6 234.7 234.8 The History of MDMA. Oxford University Press. 29 June 2023. pp. 6–16, 18, 27, 29, 32, 40. doi:10.1093/oso/9780198867364.001.0001. ISBN 978-0-19-886736-4. https://books.google.com/books?id=KSvCEAAAQBAJ&pg=PA6.
- ↑ 235.0 235.1 235.2 235.3 "The origin of MDMA ("ecstasy")--separating the facts from the myth". Die Pharmazie 61 (11): 966–972. November 2006. PMID 17152992.
- ↑ "German Patent 274350: Verfahren zur Darstellung von Alkyloxyaryl-, Dialkyloxyaryl- und Alkylendioxyarylaminopropanen bzw. deren am Stickstoff monoalkylierten Derivaten.". Kaiserliches Patentamt. 16 May 1914. http://v3.espacenet.com/publicationDetails/originalDocument?CC=DE&NR=274350C&FT=D.
- ↑ "German Patent 279194: Verfahren zur Darstellung von Hydrastinin Derivaten.". Kaiserliches Patentamt. 15 October 1914. http://v3.espacenet.com/publicationDetails/originalDocument?CC=DE&NR=279194C&FT=D.
- ↑ "History of MDMA". Ecstasy: The Clinical, Pharmacological and Neurotoxicological Effects of the Drug MDMA. Topics in the Neurosciences. 9. Boston, MA: Springer US. 1990. pp. 1–20 (2, 14). doi:10.1007/978-1-4613-1485-1_1. ISBN 978-1-4612-8799-5. http://link.springer.com/10.1007/978-1-4613-1485-1_1. Retrieved 15 May 2025.
- ↑ 239.0 239.1 "Some Relations Between Chemical Structure and Physiological Action of Mescaline and Related Compounds / Structure and Action of Phenethylamines". Neuropharmacology: Transactions of the Fourth Conference, September 25, 26, and 27, 1957, Princeton, N. J.. New York: Josiah Macy Foundation. 1959. pp. 181–268. OCLC 9802642. https://bitnest.netfirms.com/external/Books/NeuropharmacologyTrans.4.181#page=5.
- ↑ 240.0 240.1 "Subjective Reactions to Phenethylamine Hallucinogens". A Pharmacologic Approach to the Study of the Mind. Springfield: CC Thomas. 1959. pp. 238–250 (241–246). ISBN 978-0-398-04254-7. https://archive.org/details/pharmacologicapp0000univ/page/238/mode/1up.
- ↑ 241.00 241.01 241.02 241.03 241.04 241.05 241.06 241.07 241.08 241.09 241.10 "Rediscovering MDMA (ecstasy): the role of the American chemist Alexander T. Shulgin". Addiction (Abingdon, England) 105 (8): 1355–1361. August 2010. doi:10.1111/j.1360-0443.2010.02948.x. PMID 20653618.
- ↑ "Relationship of the structure of mescaline and seven analogs to toxicity and behavior in five species of laboratory animals". Toxicology and Applied Pharmacology 25 (2): 299–309. June 1973. doi:10.1016/S0041-008X(73)80016-X. PMID 4197635. Bibcode: 1973ToxAP..25..299H. http://www.erowid.org/references/refs_view.php?A=ShowDoc1&ID=639. Retrieved 19 April 2009.
- ↑ "Production of d,1-N-methyl-beta-(3,4-methylenedioxyphenyl)-isopropylamine and d,1-N-methyl-beta-(3,4-dimthoxyphenyl)-isopropylamine." (in pl). Acta Polon Pharm 17: 421–425. 1960.
- ↑ 244.0 244.1 244.2 "MDMA. Nonmedical use and intoxication". Journal of Psychoactive Drugs 18 (4): 349–354. October 1986. doi:10.1080/02791072.1986.10472368. PMID 2880950. http://www.maps.org/images/pdf/1986_siegel_1.pdf. Retrieved 11 August 2015.
- ↑ The first confirmed sample was seized and identified by Chicago Police in 1970, see "Problems in Identification of Methylenedioxy and Methoxy Amphetamines". Journal of Criminal Law, Criminology, and Police Science 63 (2): 304–312. 1972. doi:10.2307/1142315. http://www.erowid.org/references/refs_view.php?A=ShowDoc1&ID=1149. Retrieved 19 April 2009.
- ↑ 246.0 246.1 "Psychedelic Drug Called Ecstasy Gains Popularity in Manhattan Nightclubs". The New York Times. 11 December 1988. https://www.nytimes.com/1988/12/11/nyregion/psychedelic-drug-called-ecstasy-gains-popularity-in-manhattan-nightclubs.html?pagewanted=2.
- ↑ 247.0 247.1 247.2 247.3 "Professor X". Wired. September 2002. http://archive.wired.com/wired/archive/10.09/professorx.html?pg=3&topic=&topic_set=. Retrieved 4 January 2015.
- ↑ 248.0 248.1 "Drug Abuse Series: MDMA". Drug Abuse Information and Monitoring Project. April 1987. https://erowid.org/chemicals/mdma/mdma_info6.shtml.
- ↑ 249.0 249.1 249.2 249.3 249.4 249.5 249.6 249.7 "An exploration of the history and controversies surrounding MDMA and MDA". Journal of Psychoactive Drugs 33 (3): 213–221. 2001. doi:10.1080/02791072.2001.10400568. PMID 11718314.
- ↑ "Alexander 'Sasha' Shulgin". Alexander Shulgin Research Institute. http://www.shulginresearch.org/home/about/alexander-sasha-shulgin/.
- ↑ 251.0 251.1 251.2 251.3 "Chapters 12, 22". PiHKAL: A Chemical Love Story (7th printing, 1st ed.). Berkeley, CA: Transform Press. 1991. ISBN 978-0-9630096-0-9.
- ↑ "Characterization of Three New Psychotomimetics". The Psychopharmacology of Hallucinogens. New York: Pergamon Press. 1978. pp. 74–83. ISBN 978-0-08-021938-7. http://www.erowid.org/references/refs_view.php?A=ShowDocPartFrame&ID=961&DocPartID=832. Retrieved 4 January 2015.
- ↑ 253.0 253.1 253.2 253.3 "Dr. Ecstasy". The New York Times Magazine. 30 January 2005. https://www.nytimes.com/2005/01/30/magazine/30ECSTASY.html.
- ↑ 254.0 254.1 254.2 254.3 254.4 254.5 "Ecstasy Rising". Primetime Thursday (ABC News) (Special edition). 1 April 2004. http://www.thedocumentarygroup.com/PJP/Transcripts%20Files/Script_Ecstasy.doc.
- ↑ "Tribute to Jacob". The Secret Chief Revealed (2nd ed.). Sarasota, Fl: Multidisciplinary Association for Psychedelic Studies. 2004. pp. 17–18. ISBN 978-0-9660019-6-9. http://maps.org/images/pdf/books/scr/scr.pdf. Retrieved 7 January 2015.
- ↑ 256.0 256.1 "Ecstasy on Prescription". BBC Business Daily. 29 May 2018. https://www.bbc.co.uk/programmes/w3cswgvh.
- ↑ "Ten years of 'ecstasy'". Journal of the Royal Society of Medicine 92 (2): 68–72. February 1999. doi:10.1177/014107689909200206. PMID 10450215.
- ↑ 258.0 258.1 258.2 258.3 258.4 258.5 258.6 258.7 Ecstasy: The MDMA Story (Expanded 2nd ed.). Berkeley, CA: Ronin Publishing. 1994. ISBN 978-0-914171-68-3. https://books.google.com/books?id=8aqUu5M6UpwC. Retrieved 1 February 2016.
- ↑ 259.00 259.01 259.02 259.03 259.04 259.05 259.06 259.07 259.08 259.09 259.10 259.11 259.12 259.13 "The Distribution of Ecstasy". Pursuit of Ecstasy: The MDMA Experience. Albany: State Univ. of New York Press. 1994. ISBN 978-0-7914-1817-8. https://books.google.com/books?id=SwdedK36bVMC.
- ↑ 260.0 260.1 260.2 260.3 "Chapter 6: Why MDMA Should Not Have Been Made Illegal". The Drug Legalization Debate (2nd ed.). London: SAGE Publications, Inc.. 1991. ISBN 978-0-8039-3678-2. http://www.drugtext.org/pdf/Dance/party-drugs-clubbing/why-mdma-should-not-have-been-made-illegal.pdf. Retrieved 10 August 2015.
- ↑ "The Technologies of Pleasure". Altered State: The Story of Ecstasy Culture and Acid House. (Updated new ed.). London: Profile Books. 2010. ISBN 978-1-84765-641-4. https://books.google.com/books?id=fc8x9qeCekQC.
- ↑ "Countdown to Ecstasy: A New Drug for a New Millennium". The Austin Chronicle (Weekly Wire). 12 June 2000. http://www.weeklywire.com/ww/06-12-00/austin_music_feature.html.
- ↑ "Molly Isn't Who You Think She Is: A Deeper Look at MDMA". Playboy. 20 October 2013. http://www.playboy.com/articles/molly-party-drug-ecstasy. Retrieved 6 August 2015.
- ↑ "A Brief History of the Rave Scene". Trance Formation: The Spiritual and Religious Dimensions of Global Rave Culture. New York, NY: Routledge. 2005. pp. 21–22. ISBN 978-0-415-97090-7.
- ↑ "Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity". Psychopharmacology 173 (3–4): 234–241. May 2004. doi:10.1007/s00213-003-1712-7. PMID 15007594. http://psy.swansea.ac.uk/staff/parrott/p-IsEcstasyMDMA-Psychopharm-2004.pdf. Retrieved 7 August 2015.
- ↑ "MDMA on the street: Analysis Anonymous". Journal of Psychoactive Drugs 18 (4): 363–369. October 1986. doi:10.1080/02791072.1986.10472371. PMID 2880953.
- ↑ "Schedules of Controlled Substances Proposed Placement of 3,4-Methylenedioxymethamphetamine in Schedule I". Federal Register 49 (146). 27 July 1984. http://maps.org/research-archive/dea-mdma/pdf/0194.PDF. Retrieved 15 January 2015.
- ↑ "Getting High on 'Ecstasy'". Newsweek Magazine (Life/Style): p. 96. 15 April 1985. http://www.maps.org/research-archive/hmma/Newsweek4.85.pdf.
- ↑ 269.0 269.1 269.2 "The History of MDMA". Ecstasy: the complete guide; a comprehensive look at the risks and benefits of MDMA. Rochester, VT: Park Street Press. 2001. ISBN 978-0-89281-857-0.
- ↑ 270.0 270.1 "U.S. will ban 'ecstasy,' a hallucinogenic drug". The New York Times. The Associated Press. 1 June 1985. https://www.nytimes.com/1985/06/01/us/us-will-ban-ecstasy-a-hallucinogenic-drug.html.
- ↑ "MDMA – FDA REPORT, 1985". Food and Drug Administration. 1985. https://www.erowid.org/chemicals/mdma/mdma_law3.shtml.
- ↑ "DEA To Ban "Ecstasy" – The Drug MDMA". The Associated Press. 30 May 1985. https://apnews.com/26214cd32a8b4c05e361158df14715b0.
- ↑ "U.S. to Ban Use of Drug MDMA: Street Abuse Cited; Used by Psychiatrists". Los Angeles Times. 31 May 1985. https://www.latimes.com/archives/la-xpm-1985-05-31-mn-14566-story.html.
- ↑ "Alexander Shulgin, Psychedelia Researcher, Dies at 88". The New York Times. 7 June 2014. https://www.nytimes.com/2014/06/08/us/alexander-shulgin-psychedelia-researcher-dies-at-88.html?_r=0.
- ↑ "Chemist Alexander Shulgin, popularizer of the drug Ecstasy, dies at 88". The Washington Post (WP Company LLC). 3 June 2014. https://www.washingtonpost.com/national/health-science/chemist-alexander-shulgin-popularizer-of-the-drug-ecstasy-dies-at-88/2014/06/03/19fd9580-eb34-11e3-b98c-72cef4a00499_story.html.
- ↑ "Ecstasy has its pros and cons". Kokomo Tribune. Harper's Bazaar (Kokomo, Indiana): p. 6. 23 November 1985. https://newspaperarchive.com/profile/Robert_Thomas/clipnumber/34618/.

- ↑ "Lester Grinspoon, M.d., Petitioner, v. Drug Enforcement Administration, Respondent, 828 F.2d 881 (1st Cir. 1987)". US Court of Appeals for the First Circuit. https://law.justia.com/cases/federal/appellate-courts/F2/828/881/368975/.
- ↑ "Challenges with benchmarking of MDMA-assisted psychotherapy". Nature Medicine 27 (10): 1689–1690. October 2021. doi:10.1038/s41591-021-01525-0. PMID 34635857. https://hal.archives-ouvertes.fr/hal-03414583/file/Halvorsen%20et%20al%20-%202021%20-%20Challenges%20with%20benchmarking%20of%20MDMA-assisted%20psychotherapy.pdf. Retrieved 9 May 2022.
- ↑ WHO Expert Committee on Drug Dependence: Twenty-second Report.. Geneva: World Health Organization. 1985. pp. 24–25. http://whqlibdoc.who.int/trs/WHO_TRS_729.pdf. Retrieved 29 August 2012.
- ↑ "Decision to place MDMA into Schedule I". Commission on Narcotic Drugs. 11 February 1986. http://www.unodc.org/documents/commissions/CND/Drug_Resolutions/1980-1989/1986/CND_Decision-1986-07_S-IX.pdf.
- ↑ "Overdoses of 'Molly' Led to Electric Zoo Deaths". The New York Times. 12 September 2013. http://artsbeat.blogs.nytimes.com/2013/09/12/overdoses-of-molly-led-to-electric-zoo-deaths/?_r=0.
- ↑ Goldfrank's toxicologic emergencies (9th ed.). New York: McGraw-Hill Medical. 2011. ISBN 978-0-07-160593-9.
- ↑ "Bibliography of Psychedelic Research Studies.". Multidisciplinary Association for Psychedelic Studies (MAPS). Santa Cruz, CA. http://www.maps.org/research/.
- ↑ "What Is Molly and Why Is It Dangerous?". NBCNews.com. 23 February 2015. https://www.nbcnews.com/health/health-news/what-molly-why-it-dangerous-n311291. "Why is it called Molly? That's short for "molecule." "You can put a ribbon and bow on it and call it a cute name like 'Molly' and people are all in," said Paul Doering, professor emeritus of pharmacology at the University of Florida."
- ↑ "Molly: Pure, but Not So Simple". The New York Times. 21 June 2013. https://www.nytimes.com/2013/06/23/fashion/molly-pure-but-not-so-simple.html.
- ↑ "Mephedrone (4-Methylmethcathinone) appearing in "Ecstasy" in the Netherlands". 19 September 2010. http://scientopia.org/blogs/drugmonkey/2010/09/19/mephedrone-4-methylmethcathinone-appearing-in-ecstasy-in-the-netherlands/.
- ↑ "Why ecstasy is 'vanishing' from UK nightclubs". BBC News. 19 January 2010. https://news.bbc.co.uk/2/hi/uk_news/england/london/8468372.stm.
- ↑ "Watch Out for Pentylone, the Horrible New MDMA Additive". Vice. Aug 4, 2017. https://www.vice.com/en/article/watch-out-for-pentylone-the-horrible-new-mdma-additive/. Retrieved 31 May 2018.
- ↑ "MDMA may have protected Nova attack survivors from trauma, study suggests". 2025-03-07. https://www.bbc.com/news/articles/c9wpy14wyd0o.
- ↑ "MDMA and LSD may have helped October 7 survivors". 2025-03-08. https://www.news.com.au/world/middle-east/lsd-mdma-party-drugs-may-have-helped-survivors-of-october-7-attack-study-claims/news-story/361e7726844087d6d023f010bccf1c48.
- ↑ "Annual prevalence of use of drugs, by region and globally, 2016". World Drug Report 2018. United Nations Office on Drugs and Crime. 2018. https://dataunodc.un.org/drugs/prevalence_regional. Retrieved 7 July 2018.
- ↑ "MDMA and psilocybin: What GPs need to know". Newsgp. https://www1.racgp.org.au/newsgp/gp-opinion/mdma-and-psilocybin-what-gps-need-to-know#:~:text=It%20means%20psilocybin%20and%20MDMA,restricts%20supply%20to%20clinical%20trials..
- ↑ "Is psychiatry ready for medical MDMA?" (in en-US). 2018-03-29. http://theconversation.com/is-psychiatry-ready-for-medical-mdma-94105.
- ↑ "Misuse of Drugs Act 1981". The Government of Western Australia. 23 October 1981. https://www.slp.wa.gov.au/legislation/statutes.nsf/main_mrtitle_4607_homepage.html.
- ↑ "ACT government decriminalises small amounts of illicit drugs including speed, heroin and cocaine". Australian Broadcasting Corporation. 20 October 2022. https://www.abc.net.au/news/2022-10-20/act-decriminalises-small-amounts-of-illicit-drugs-heroin-cocaine/101552008.
- ↑ "The ACT has today decriminalised small amounts of some illicit drugs. But what does that mean?". 27 October 2023. https://www.abc.net.au/news/2023-10-28/canberra-drug-decriminalisation-laws-begin-today/103032128.
- ↑ "Schedule I". Controlled Drugs and Substances Act. Isomer Design. http://isomerdesign.com/Cdsa/schedule.php?schedule=1§ion=18.5&structure=C.
- ↑ "Definitions and interpretations". Controlled Drugs and Substances Act. Isomer Design. http://isomerdesign.com/Cdsa/definitions.php?structure=C.
- ↑ "Decriminalizing people who use drugs in B.C.". Government Communications and Public Engagement. https://www2.gov.bc.ca/gov/content/overdose/decriminalization.
- ↑ "B.C. recorded 211 toxic drug deaths — almost 7 a day — in January, coroner reports". CBC.ca. March 7, 2023. https://www.cbc.ca/news/canada/british-columbia/bc-toxic-drugs-deaths-january-2023-1.6770643.
- ↑ "Valtioneuvoston asetus huumausaineina pidettävistä aineista, valmisteista ja kasveista | 543/2008 | Lainsäädäntö | Finlex". https://www.finlex.fi/fi/lainsaadanto/2008/543.
- ↑ "KKO:2005:56 | 11.5.2005 | Ennakkopäätökset | Korkein oikeus | Finlex". https://finlex.fi/fi/oikeuskaytanto/korkein-oikeus/ennakkopaatokset/2005/56.
- ↑ "MDMA /// Beyond Ecstasy - Report - Government.nl" (in en-GB). 2024-05-31. https://www.government.nl/documents/reports/2024/05/31/mdma-beyond-ecstasy.
- ↑ 304.0 304.1 "An Examination of Federal Sentencing Guidelines' Treatment of MDMA ('Ecstasy')". Belmont Law Review 1: 267. 2014.
- ↑ "Rapport Drugs in Lijsten". Rijksoverheid.nl. 27 June 2011. http://www.rijksoverheid.nl/documenten-en-publicaties/rapporten/2011/06/27/rapport-drugs-in-lijsten.html.
- ↑ "Committee: the current system of the Opium Act does not have to be changed". 24 June 2011. http://www.government.nl/documents-and-publications/press-releases/2011/06/24/committee-the-current-system-of-the-opium-act-does-not-have-to-be-changed.html.
- ↑ (epub file) Drugs 2.0: the web revolution that's changing how the world gets high. London: Portobello. 2013. ISBN 978-1-84627-459-6. https://books.google.com/books?id=gj6zMQEACAAJ&q=Drugs+2.0.
- ↑ "Misuse of Drugs Act 1971". Statutelaw.gov.uk. 5 January 1998. http://www.statutelaw.gov.uk/content.aspx?LegType=All+Primary&PageNumber=57&NavFrom=2&parentActiveTextDocId=1367412&ActiveTextDocId=1367472&filesize=411.
- ↑ "Ecstasy 'no more dangerous than horse riding'". 7 February 2009. https://www.telegraph.co.uk/news/uknews/law-and-order/4537874/Ecstasy-no-more-dangerous-than-horse-riding.html.
- ↑ "Equasy-- an overlooked addiction with implications for the current debate on drug harms". Journal of Psychopharmacology (Oxford, England) 23 (1): 3–5. January 2009. doi:10.1177/0269881108099672. PMID 19158127.
- ↑ "Why Professor David Nutt was shown the door". The Guardian (London). 2 November 2009. https://www.theguardian.com/politics/2009/nov/02/drug-policy-alan-johnson-nutt.
- ↑ Schedules of Controlled Substances; Scheduling of 3,4-Methylenedioxymethamphetamine (MDMA) Into Schedule I of the Controlled Substances Act; Remand, 53 Fed. Reg. 5,156 (DEA 22 February 1988).
- ↑ "Court Rejects Harsh Federal Drug Sentencing Guideline as Scientifically Unjustified". American Civil Liberties Union. 15 July 2011. https://www.aclu.org/criminal-law-reform/court-rejects-harsh-federal-drug-sentencing-guideline-scientifically-unjustified.
- ↑ 314.0 314.1 "Statistical Bulletin 2018 — prevalence of drug use". European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). http://www.emcdda.europa.eu/data/stats2018/gps_en.
- ↑ "Ecstasy use among US adolescents from 1999 to 2008". Drug and Alcohol Dependence 112 (1–2): 33–38. November 2010. doi:10.1016/j.drugalcdep.2010.05.006. PMID 20570447.
- ↑ Annual report: the state of the drugs problem in Europe. Luxembourg: Office for Official Publications of the European Communities. 2008. p. 49. ISBN 978-92-9168-324-6. http://www.emcdda.europa.eu/attachements.cfm/att_64227_EN_EMCDDA_AR08_en.pdf. Retrieved 1 December 2008.
- ↑ "Ecstasy: high purity powder available". European Drug Report. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). 2014. p. 26. doi:10.2810/32306. ISBN 978-92-9168-694-0. http://www.emcdda.europa.eu/attachements.cfm/att_228272_EN_TDAT14001ENN.pdf. Retrieved 4 June 2014.
- ↑ "Ecstasy-type substances Retail and wholesale prices* and purity levels, by drug, region and country or territory". United Nations Office on Drugs and Crime. http://www.unodc.org/documents/wdr2014/Statistics/Prices_ATS.xls.
- ↑ "Monitoring of illicit pill distribution networks using an image collection exploration framework". Forensic Science International 223 (1–3): 298–305. November 2012. doi:10.1016/j.forsciint.2012.10.004. PMID 23107059. http://www.fsijournal.org/article/S0379-0738(12)00458-6/abstract. Retrieved 9 December 2013.
- ↑ "10 years of ecstasy and other party drug use in Australia: What have we done and what is there left to do?". Drugtext.org. http://www.drugtext.org/library/articles/dillon.htm.
- ↑ "Erowid MDMA Vault: Images". https://www.erowid.org/chemicals/mdma/mdma_images.shtml.
- ↑ "Now sick dealers peddle Shaun the Sheep Ecstasy tablets". Western Daily Press. 31 July 2015. http://www.westerndailypress.co.uk/sick-dealers-peddle-Shaun-Sheep-Ecstasy-tablets/story-27521142-detail/story.html.
- ↑ "A Systematic Review of the MDMA Model to Address Social Impairment in Autism". Current Neuropharmacology 19 (7): 1101–1154. 2021-07-01. doi:10.2174/1570159X19666210101130258. PMID 33388021.
- ↑ "Alternative Approaches: The Good, the Bad and the Worrying: Psychedelics for Depression". Chemically Imbalanced: The Making and Unmaking of the Serotonin Myth. Flint. 16 January 2025. ISBN 978-1-80399-680-6. https://books.google.com/books?id=e0MkEQAAQBAJ. Retrieved 16 October 2025.
External links
- A Multi-Site Phase 3 Study of MDMA-Assisted Therapy for PTSD (MAPP2)
- "MDMA Facts and Statistics". National Institute on Drug Abuse. 15 June 2020. https://www.drugabuse.gov/publications/drugfacts/mdma-ecstasymolly.