Chemistry:Esfenvalerate
From HandWiki
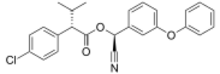
| |
| Names | |
|---|---|
| Preferred IUPAC name
(S)-Cyano(3-phenoxyphenyl)methyl (2S)-2-(4-chlorophenyl)-3-methylbutanoate | |
| Other names
Asana
(S)-Fenvalerate | |
| Identifiers | |
3D model (JSmol)
|
|
| 4275674 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| UN number | 3349 |
| |
| |
| Properties | |
| C25H22ClNO3 | |
| Molar mass | 419.91 g·mol−1 |
| Density | 1.211 g/cm3 |
| Melting point | 60 °C (140 °F; 333 K) |
| log P | 6.22 |
| Vapor pressure | 0 mmHg at 25 °C |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H301, H313, H316, H317, H320, H330, H335, H370, H373, H410 | |
| P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+310, P302+352, P304+340, P305+351+338, P307+311, P310, P312, P314, P320, P321, P330, P332+313, P333+313, P337+313, P363, P391 | |
| Flash point | 256 °C (493 °F; 529 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Esfenvalerate is a synthetic pyrethroid insecticide marketed under the brand Asana.[2] It is the (S)-enantiomer of fenvalerate.[3]
In the United States, a limit of .05 ppm of the chemical's residue is permissible in food.[4]
References
- ↑ Kelly, Kevin. "Environmental Fate of Esfenvalerate". California Environmental Protection Agency. https://docs.google.com/viewer?a=v&q=cache:Dj4zTYgWkVwJ:www.cdpr.ca.gov/docs/emon/pubs/fatememo/esfen.pdf+&hl=en&gl=us&pid=bl&srcid=ADGEESioBjkb-ZFR_RL4b81PgVXQtE1uuVNiP1oizd-B5RHIElqfOsbh11O7x5n5U7QO5X_Ds0xSwaqa_hO3sr8ipOGsoy7zWrd3ZHnpjvZvb9hV4Ot9XQeZEzZZKUmLOeC13D3sii_j&sig=AHIEtbT0y5uiRpRhAiBnZ4nP9zihtQ56GA.
- ↑ Fishel, Frederick M. (2012). "Pesticide Toxicity Profile: Synthetic Pyrethroid Pesticides". University of Florida. http://edis.ifas.ufl.edu/pi091.
- ↑ "Esfenvalerate". EXTONET (Extension Toxicology Network). Cooperative Extension Offices of Cornell University, Michigan State University, Oregon State University, and University of California at Davis. May 1994. http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/esfenvalerate-ext.html.
- ↑ The Code of Federal Regulations of the United States of America. U.S. Government Printing Office. 2006. pp. 445–446. https://books.google.com/books?id=ifk6AAAAIAAJ&q=%22%28S%29-cyano+%283-phenoxyphenyl%29+methyl-%28S%29-4-chloro-alpha-%281-methylethyl%29+benzeneacetate%22&pg=PA446.
