Chemistry:Fexinidazole
 | |
| Clinical data | |
|---|---|
| Other names |
|
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
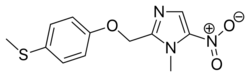
| Formula | C12H13N3O3S |
| Molar mass | 279.31 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fexinidazole is a medication used to treat African trypanosomiasis (sleeping sickness) caused by Trypanosoma brucei gambiense.[3] It is effective against both first and second stage disease.[3] Also a potential new treatment for Chagas disease, a neglected tropical disease that affects millions of people worldwide.[4] It is taken by mouth.[5]
Common side effects include nausea, vomiting, headache, and trouble sleeping.[6] Other side effects may include QT prolongation, psychosis, and low white blood cells.[7] It is unclear if use during pregnancy or breast feeding is safe.[7] Fexinidazole is in the antiparasitic and the nitroimidazole family of medications.[5] It is believed to work by turning on certain enzymes within the parasites that result in their death.[6]
Fexinidazole was first described in 1978.[8] It was given a positive opinion by the European Medicines Agency in 2018.[6] It is on the World Health Organization's List of Essential Medicines.[9][10] Development for sleeping sickness was funded by the Drugs for Neglected Diseases initiative in collaboration with Sanofi.[11] Fexinidazole was approved for medical use in the United States in July 2021.[1]
Medical use
Sleeping sickness
A trial in Africa found fexinidazole to be 91% effective at treating sleeping sickness.[6][12] Though less effective than nifurtimox with eflornithine in severe disease, fexinidazole has the benefit that it can be taken by mouth.[6]
Fexinidazole is the first drug candidate for the treatment of advanced-stage sleeping sickness in thirty years.[13]
Efficacy and safety
In cell culture, fexinidazole has an IC50 of around 1–4μM against Trypanosoma brucei.[14] In the mouse model, fexinidazole cures both the first, haemolymphatic, and the second, meningoencephalitic stage of the infection, the latter at 100 mg/kg twice daily for 5 days. In patients, the clinical trials managed by DNDi and supported by Swiss TPH mainly conducted in the Democratic Republic of the Congo demonstrated that oral fexinidazole is safe and effective for use against first- and early second-stage sleeping sickness. Based on the positive opinion issued by the European Medicines Agency in 2018, the WHO has released new interim guidelines for the treatment of HAT including fexinidazole as the new therapy for first-stage and non-severe second-stage sleeping sickness caused by Trypanosoma brucei gambiense (gHAT) [15] Recently, a study of the safety and efficacy of oral fexinidazole in children with gambiense human African trypanosomiasis was accomplished and concluded that orally administered fexinidazole showed high efficacy across all stages of gambiense human African trypanosomiasis infection in children aged 6 years and older and weighing more than 20 kg. The benefit-to-risk ratio of fexinidazole for treating children with gambiense human African trypanosomiasis, regardless of disease stage, is positive. Current interventions for diagnosing, staging, and treating gambiense human African trypanosomiasis require resources, trained personnel, equipment, and hospital infrastructure. These potentially costly procedures are therefore difficult to implement in remote areas or in those that might be mired in conflict, which could prevent the goal of eliminating gambiense human African trypanosomiasis by 2030.25,26 Simplified oral treatments such as fexinidazole or single-dose oral treatments such as acoziborole (currently in clinical trials)27 that can cure both disease stages of gambiense human African trypanosomiasis and circumvent the need for systematic disease staging with lumbar puncture (a procedure associated with complications and anxiety, particularly in children28) would benefit both patients and health-care professionals [16] Furthermore, Damasio et al. evaluated the in vivo oral efficacy of self-emulsifying drug delivery systems (SEDDS) containing fexinidazole (FEX) in the experimental treatment of visceral leishmaniasis (VL). The developed FEX-SEDDS formulation presented as a clear, yellowish liquid without precipitate. In the simulated gastric and intestinal media, the FEX-SEDDS had a size of 97 ± 1 and 106 ± 9 nm, respectively. The FEX retention in droplets after SEDDS dilution in simulated gastrointestinal media was almost 100%. Antileishmanial efficacy studies showed that FEX-SEDDS was the only treatment able to significantly (p < 0.05) reduce the parasite burden in the liver and spleen of animals experimentally infected with Leishmania infantum [17]
Mechanism of action
The biologically relevant active metabolites in vivo are the sulfoxide and sulfone.[18][19]
Synthesis
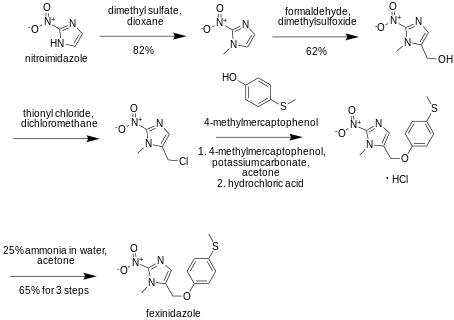
History
Fexinidazole was discovered by the German pharmaceutical company Hoechst AG, but its development as a pharmaceutical was halted in the 1980s.[21]
The US Food and Drug Administration granted the application for fexinidazole orphan drug designation.[22]
Society and culture
Fexinidazole Winthrop, a Sanofi-Aventis product developed with the Drugs for Neglected Diseases Initiative (DNDi), received a positive endorsement from the European Medicines Agency in 2018, for use in non-European markets.[23][24] It was approved for the treatment of Trypanosoma brucei gambiense human African trypanosomiasis (HAT) in the Democratic Republic of the Congo (DRC) in December 2018.[25] Fexinidazole was included in the 'role of honour' in Préscrire magazine's 2020 prize list.Fexinidazole Winthrop° (fexinidazole) : au Tableau d'Honneur 2020
Veterinary use
Fexinidazole is promising in African animal trypanosomiasis. Torreele et al. 2010 found the drug to be effective against T. b. gambiense infection of mice, rats, rabbits and beagles. They also found no toxicity in any of them, including a lack of mutagenicity despite in vitro mutagenicity.[26]
References
- ↑ 1.0 1.1 "Fexinidazole: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=214429.
- ↑ "Fexinidazole tablet". DailyMed. U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=74a611bc-9977-46bb-b626-0370b3031628.
- ↑ 3.0 3.1 "Fexinidazole Winthrop H-W-2320" (in en). 22 January 2019. https://www.ema.europa.eu/en/fexinidazole-winthrop-h-w-2320.
- ↑ "A Phase 2, Randomized, Multicenter, Placebo-Controlled, Proof-of-Concept Trial of Oral Fexinidazole in Adults With Chronic Indeterminate Chagas Disease". Clinical Infectious Diseases 76 (3): e1186–e1194. February 2023. doi:10.1093/cid/ciac579. PMID 35925555.
- ↑ 5.0 5.1 "Fexinidazole: First Global Approval". Drugs 79 (2): 215–220. February 2019. doi:10.1007/s40265-019-1051-6. PMID 30635838.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Fexinidazole Winthrop (fexinidazole)". https://www.ema.europa.eu/en/documents/medicine-outside-eu/fexinidazole-winthrop-summary-public_en.pdf.
- ↑ 7.0 7.1 "Fexinidazole Winthrop". https://www.ema.europa.eu/en/documents/medicine-outside-eu/fexinidazole-winthrop-product-information_en.pdf.
- ↑ "Antileishmanial Drug Discovery: Past, Present, and Future Perspectives" (in en). Drug Discovery for Leishmaniasis. Royal Society of Chemistry. 2017. p. 30. ISBN 9781788012584. https://books.google.com/books?id=q7WrDwAAQBAJ&pg=PA30.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "Fexinidazole – DNDi". 31 December 2004. https://www.dndi.org/achievements/fexinidazole/.
- ↑ "Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial". Lancet 391 (10116): 144–154. January 2018. doi:10.1016/s0140-6736(17)32758-7. PMID 29113731.
- ↑ Boelaert, Marleen, ed (December 2010). "Fexinidazole--a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness". PLOS Neglected Tropical Diseases 4 (12): e923. doi:10.1371/journal.pntd.0000923. PMID 21200426.
- ↑ "Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness". Antimicrobial Agents and Chemotherapy (American Society for Microbiology) 55 (12): 5602–5608. December 2011. doi:10.1128/aac.00246-11. PMID 21911566.
- ↑ "Fexinidazole for Human African Trypanosomiasis, the Fruit of a Successful Public-Private Partnership". Diseases 10 (4): 90. October 2022. doi:10.3390/diseases10040090. PMID 36278589.
- ↑ "Safety and efficacy of oral fexinidazole in children with gambiense human African trypanosomiasis: a multicentre, single-arm, open-label, phase 2-3 trial". The Lancet. Global Health 10 (11): e1665–e1674. November 2022. doi:10.1016/S2214-109X(22)00338-2. PMID 36179736.
- ↑ "A new oral self-emulsifying drug delivery system improves the antileishmania efficacy of fexinidazole in vivo". International Journal of Pharmaceutics 631: 122505. January 2023. doi:10.1016/j.ijpharm.2022.122505. PMID 36549405.
- ↑ "The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis". Science Translational Medicine 4 (119): 119re1. February 2012. doi:10.1126/scitranslmed.3003326. PMID 22301556.
- ↑ "Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis". Antimicrobial Agents and Chemotherapy 54 (7): 2893–2900. July 2010. doi:10.1128/AAC.00332-10. PMID 20439607.
- ↑ "Synthetic Approaches to the New Drugs Approved During 2021". Journal of Medicinal Chemistry (American Chemical Society (ACS)) 66 (15): 10150–10201. August 2023. doi:10.1021/acs.jmedchem.3c00501. PMID 37528515.
- ↑ "Jump-Start on Slow Trek to Treatment for a Disease". The New York Times. 8 January 2008. https://www.nytimes.com/2008/01/08/health/research/08slee.html.
- ↑ (PDF) Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (Report). 13 May 2022. https://www.fda.gov/media/155227/download. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "CHMP Summary of Opinion - Fexinidazole Winthrop". https://www.ema.europa.eu/documents/smop-initial/chmp-summary-opinion-fexinidazole-winthrop_en.pdf.
- ↑ "Rapid Cure Approved for Sleeping Sickness, a Horrific Illness". The New York Times. 16 November 2018. https://www.nytimes.com/2018/11/16/health/sleeping-sickness-africa-cure.html.
- ↑ "Fexinidazole, the first all-oral treatment for sleeping sickness, approved in Democratic Republic of Congo". 29 January 2019. https://www.dndi.org/2019/media-centre/press-releases/fexinidazole-sleeping-sickness-approved-democratic-republic-congo/.
- ↑ "Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness)". The Lancet. Neurology (Elsevier) 12 (2): 186–194. February 2013. doi:10.1016/s1474-4422(12)70296-x. PMID 23260189.
External links
- "Fexinidazole". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/fexinidazole.
 |

