Chemistry:Nitazoxanide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Alinia, Nizonide, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603017 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Antiprotozoal Broad-spectrum antiparasitic Broad-spectrum antiviral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Nitazoxanide: ? Tizoxanide: over 99%[1][2] |
| Metabolism | Rapidly hydrolyzed to tizoxanide[1] |
| Metabolites | tizoxanide[1][2] tizoxanide glucuronide[1][2] |
| Elimination half-life | 3.5 hours[3] |
| Excretion | Kidney, bile duct, and fecal[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
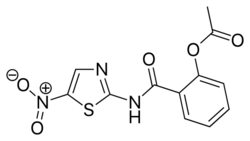
| Formula | C12H9N3O5S |
| Molar mass | 307.28 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Nitazoxanide, sold under the brand name Alinia among others, is a broad-spectrum antiparasitic and broad-spectrum antiviral medication that is used in medicine for the treatment of various helminthic, protozoal, and viral infections.[4][5][6] It is indicated for the treatment of infection by Cryptosporidium parvum and Giardia lamblia in immunocompetent individuals and has been repurposed for the treatment of influenza.[1][6] Nitazoxanide has also been shown to have in vitro antiparasitic activity and clinical treatment efficacy for infections caused by other protozoa and helminths;[4][7] evidence (As of 2014) suggested that it possesses efficacy in treating a number of viral infections as well.[6]
Chemically, nitazoxanide is the prototype member of the thiazolides, a class of drugs which are synthetic nitrothiazolyl-salicylamide derivatives with antiparasitic and antiviral activity.[4][6][8] Tizoxanide, an active metabolite of nitazoxanide in humans, is also an antiparasitic drug of the thiazolide class.[4][9]
Nitazoxanide tablets were approved as a generic medication in the United States in 2020.[10]
Uses
Nitazoxanide is an effective first-line treatment for infection by Blastocystis species[11][12] and is indicated for the treatment of infection by Cryptosporidium parvum or Giardia lamblia in immunocompetent adults and children.[1] It is also an effective treatment option for infections caused by other protozoa and helminths (e.g., Entamoeba histolytica,[13] Hymenolepis nana,[14] Ascaris lumbricoides,[15] and Cyclospora cayetanensis[16]).[7]
Chronic hepatitis B
Nitazoxanide alone has shown preliminary evidence of efficacy in the treatment of chronic hepatitis B over a one-year course of therapy.[17] Nitazoxanide 500 mg twice daily resulted in a decrease in serum HBV DNA in all of 4 HBeAg-positive patients, with undetectable HBV DNA in 2 of 4 patients, loss of HBeAg in 3 patients, and loss of HBsAg in one patient. Seven of 8 HBeAg-negative patients treated with nitazoxanide 500 mg twice daily had undetectable HBV DNA and 2 had loss of HBsAg. Additionally, nitazoxanide monotherapy in one case and nitazoxanide plus adefovir in another case resulted in undetectable HBV DNA, loss of HBeAg and loss of HBsAg.[18] These preliminary studies showed a higher rate of HBsAg loss than any currently licensed therapy for chronic hepatitis B. The similar mechanism of action of interferon and nitazoxanide suggest that stand-alone nitazoxanide therapy or nitazoxanide in concert with nucleos(t)ide analogs have the potential to increase loss of HBsAg, which is the ultimate end-point of therapy. A formal phase 2 study is being planned for 2009.[19]
Chronic hepatitis C
Romark initially decided to focus on the possibility of treating chronic hepatitis C with nitazoxanide.[20] The drug garnered interest from the hepatology community after three phase II clinical trials involving the treatment of hepatitis C with nitazoxanide produced positive results for treatment efficacy and similar tolerability to placebo without any signs of toxicity.[20] A meta-analysis from 2014 concluded that the previous held trials were of low-quality and withheld with a risk of bias. The authors concluded that more randomized trials with low risk of bias are needed to determine if Nitazoxanide can be used as an effective treatment for chronic hepatitis C patients.[21]
Contraindications
Nitazoxanide is contraindicated only in individuals who have experienced a hypersensitivity reaction to nitazoxanide or the inactive ingredients of a nitazoxanide formulation.[1]
Adverse effects
The side effects of nitazoxanide do not significantly differ from a placebo treatment for giardiasis;[1] these symptoms include stomach pain, headache, upset stomach, vomiting, discolored urine, excessive urinating, skin rash, itching, fever, flu syndrome, and others.[1][22] Nitazoxanide does not appear to cause any significant adverse effects when taken by healthy adults.[1][2]
Overdose
Information on nitazoxanide overdose is limited. Oral doses of 4 grams in healthy adults do not appear to cause any significant adverse effects.[1][2] In various animals, the oral LD50 is higher than 10 g/kg.[1]
Interactions
Due to the exceptionally high plasma protein binding (>99.9%) of nitazoxanide's metabolite, tizoxanide, the concurrent use of nitazoxanide with other highly plasma protein-bound drugs with narrow therapeutic indices (e.g., warfarin) increases the risk of drug toxicity.[1] In vitro evidence suggests that nitazoxanide does not affect the CYP450 system.[1]
Pharmacology
Pharmacodynamics
The anti-protozoal activity of nitazoxanide is believed to be due to interference with the pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron-transfer reaction that is essential to anaerobic energy metabolism.[1][8] PFOR inhibition may also contribute to its activity against anaerobic bacteria.[23]
It has also been shown to have activity against influenza A virus in vitro.[24] The mechanism appears to be by selectively blocking the maturation of the viral hemagglutinin at a stage preceding resistance to endoglycosidase H digestion. This impairs hemagglutinin intracellular trafficking and insertion of the protein into the host plasma membrane.[citation needed]
Nitazoxanide modulates a variety of other pathways in vitro, including glutathione-S-transferase and glutamate-gated chloride ion channels in nematodes, respiration and other pathways in bacteria and cancer cells, and viral and host transcriptional factors.[23]
Pharmacokinetics
Following oral administration, nitazoxanide is rapidly hydrolyzed to the pharmacologically active metabolite, tizoxanide, which is 99% protein bound.[1][9] Tizoxanide is then glucuronide conjugated into the active metabolite, tizoxanide glucuronide.[1] Peak plasma concentrations of the metabolites tizoxanide and tizoxanide glucuronide are observed 1–4 hours after oral administration of nitazoxanide, whereas nitazoxanide itself is not detected in blood plasma.[1]
Roughly 2⁄3 of an oral dose of nitazoxanide is excreted as its metabolites in feces, while the remainder of the dose excreted in urine.[1] Tizoxanide is excreted in the urine, bile and feces.[1] Tizoxanide glucuronide is excreted in urine and bile.[1]
Chemistry
Nitazoxanide is the prototype member of the thiazolides, which is a drug class of structurally-related broad-spectrum antiparasitic compounds.[4] Nitazoxanide is a light yellow crystalline powder. It is poorly soluble in ethanol and practically insoluble in water.[citation needed]
History
Nitazoxanide was originally discovered in the 1980s by Jean-François Rossignol at the Pasteur Institute. Initial studies demonstrated activity versus tapeworms. In vitro studies demonstrated much broader activity. Dr. Rossignol co-founded Romark Laboratories, with the goal of bringing nitazoxanide to market as an anti-parasitic drug. Initial studies in the USA were conducted in collaboration with Unimed Pharmaceuticals, Inc. (Marietta, GA) and focused on development of the drug for treatment of cryptosporidiosis in AIDS. Controlled trials began shortly after the advent of effective anti-retroviral therapies. The trials were abandoned due to poor enrollment and the FDA rejected an application based on uncontrolled studies.[citation needed]
Subsequently, Romark launched a series of controlled trials. A placebo-controlled study of nitazoxanide in cryptosporidiosis demonstrated significant clinical improvement in adults and children with mild illness. Among malnourished children in Zambia with chronic cryptosporidiosis, a three-day course of therapy led to clinical and parasitologic improvement and improved survival. In Zambia and in a study conducted in Mexico, nitazoxanide was not successful in the treatment of cryptosporidiosis in advanced infection with human immunodeficiency virus at the doses used. However, it was effective in patients with higher CD4 counts. In treatment of giardiasis, nitazoxanide was superior to placebo and comparable to metronidazole. Nitazoxanide was successful in the treatment of metronidazole-resistant giardiasis. Studies have suggested efficacy in the treatment of cyclosporiasis, isosporiasis, and amebiasis.[25] Recent studies have also found it to be effective against beef tapeworm(Taenia saginata).[18]
Pharmaceutical products
Dosage forms
Nitazoxanide is currently available in two oral dosage forms: a tablet (500 mg) and an oral suspension (100 mg per 5 ml when reconstituted).[1]
An extended release tablet (675 mg) has been used in clinical trials for chronic hepatitis C; however, this form is not currently marketed or available for prescription.[20]
Brand names
Nitazoxanide is sold under the brand names Adonid, Alinia, Allpar, Annita, Celectan, Colufase, Daxon, Dexidex, Diatazox, Kidonax, Mitafar, Nanazoxid, Parazoxanide, Netazox, Niazid, Nitamax, Nitax, Nitaxide, Nitaz, Nizonide, NT-TOX, Pacovanton, Paramix, Toza, and Zox.[citation needed]
Research
(As of September 2015), nitazoxanide was in phase 3 clinical trials for the treatment influenza due to its inhibitory effect on a broad range of influenza virus subtypes and efficacy against influenza viruses that are resistant to neuraminidase inhibitors like oseltamivir.[6][26] Nitazoxanide is also being researched as a potential treatment for COVID-19,[27] chronic hepatitis B, chronic hepatitis C, rotavirus and norovirus gastroenteritis.[6]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 "Alinia- nitazoxanide tablet Alinia- nitazoxanide powder, for suspension". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=56b1575a-dff4-4c5a-a159-2f858e7a0cb8.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses". International Journal of Clinical Pharmacology and Therapeutics 40 (5): 213–220. May 2002. doi:10.5414/cpp40213. PMID 12051573.
- ↑ "Nitazoxanide". PubChem. National Center for Biotechnology Information, U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/nitazoxanide.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Research perspective: potential role of nitazoxanide in ovarian cancer treatment. Old drug, new purpose?". Cancers 5 (3): 1163–1176. September 2013. doi:10.3390/cancers5031163. PMID 24202339. "Nitazoxanide [NTZ: 2-acetyloxy-N-(5-nitro-2-thiazolyl)benzamide] is a thiazolide antiparasitic agent with excellent activity against a wide variety of protozoa and helminths. ... Nitazoxanide (NTZ) is a main compound of a class of broad-spectrum anti-parasitic compounds named thiazolides. It is composed of a nitrothiazole-ring and a salicylic acid moiety which are linked together by an amide bond ... NTZ is generally well tolerated, and no significant adverse events have been noted in human trials [13]. ... In vitro, NTZ and tizoxanide function against a wide range of organisms, including the protozoal species Blastocystis hominis, C. parvum, Entamoeba histolytica, G. lamblia and Trichomonas vaginalis [13]".
- ↑ "Nitazoxanide: a new broad spectrum antiparasitic agent". Expert Review of Anti-Infective Therapy 2 (1): 43–49. February 2004. doi:10.1586/14787210.2.1.43. PMID 15482170.
- ↑ 7.0 7.1 "Nitazoxanide: a review of its use in the treatment of gastrointestinal infections". Drugs 67 (13): 1947–1967. 2007. doi:10.2165/00003495-200767130-00015. PMID 17722965. "Nitazoxanide is effective in the treatment of protozoal and helminthic infections ... Nitazoxanide is a first-line choice for the treatment of illness caused by C. parvum or G. lamblia infection in immunocompetent adults and children, and is an option to be considered in the treatment of illnesses caused by other protozoa and/or helminths.".
- ↑ 8.0 8.1 "Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori". Antimicrobial Agents and Chemotherapy 46 (7): 2116–2123. July 2002. doi:10.1128/aac.46.7.2116-2123.2002. PMID 12069963. "Nitazoxanide (NTZ) is a redox-active nitrothiazolyl-salicylamide".
- ↑ 9.0 9.1 "Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication". Antiviral Research 77 (1): 56–63. January 2008. doi:10.1016/j.antiviral.2007.08.005. PMID 17888524.
- ↑ "First Generic Drug Approvals". https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals.
- ↑ "Blastocystis: Resources for Health Professionals". United States Centers for Disease Control and Prevention. 2017-05-02. https://www.cdc.gov/parasites/blastocystis/health_professionals/.
- ↑ "Update on the pathogenic potential and treatment options for Blastocystis sp". Gut Pathogens 6: 17. May 2014. doi:10.1186/1757-4749-6-17. PMID 24883113. PMC 4039988. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4039988/table/T2/. "Blastocystis is one of the most common intestinal protists of humans. ... A recent study showed that 100% of people from low socio-economic villages in Senegal were infected with Blastocystis sp. suggesting that transmission was increased due to poor hygiene sanitation, close contact with domestic animals and livestock, and water supply directly from well and river [10]. ...".
- ↑ "Parasitic infections in solid-organ transplant recipients". Current Opinion in Organ Transplantation 16 (6): 565–575. December 2011. doi:10.1097/MOT.0b013e32834cdbb0. PMID 22027588. "Nitazoxanide: intestinal amoebiasis: 500 mg po bid x 3 days".
- ↑ "Hymenolepiasis: Resources for Health Professionals". United States Centers for Disease Control and Prevention. 2017-05-02. https://www.cdc.gov/parasites/hymenolepis/health_professionals/.
- ↑ "Ascaris lumbricoides: an overview of therapeutic targets". Infectious Disorders Drug Targets 10 (5): 349–367. October 2010. doi:10.2174/187152610793180876. PMID 20701574. "new anthelmintic alternatives such as tribendimidine and Nitazoxanide have proved to be safe and effective against A. lumbricoides and other soil-transmitted helminthiases in human trials.".
- ↑ Shoff WH (5 October 2015). "Cyclospora Medication". WebMD. http://emedicine.medscape.com/article/236105-medication. "Nitazoxanide, a 5-nitrothiazole derivative with broad-spectrum activity against helminths and protozoans, has been shown to be effective against C cayetanensis, with an efficacy 87% by the third dose (first, 71%; second 75%). Three percent of patients had minor side effects."
- ↑ "Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children". International Journal of Infectious Diseases 13 (4): 518–523. July 2009. doi:10.1016/j.ijid.2008.09.014. PMID 19070525.
- ↑ 18.0 18.1 "Successful treatment of niclosamide- and praziquantel-resistant beef tapeworm infection with nitazoxanide". International Journal of Infectious Diseases 12 (1): 80–82. January 2008. doi:10.1016/j.ijid.2007.04.017. PMID 17962058.
- ↑ World Journal of Gastroenterology 2009 April 21, Emmet B Keeffe MD, Professor, Jean-François Rossignol The Romark Institute for Medical Research, Tampa
- ↑ 20.0 20.1 20.2 "Treatment of chronic viral hepatitis with nitazoxanide and second generation thiazolides". World Journal of Gastroenterology 15 (15): 1805–1808. April 2009. doi:10.3748/wjg.15.1805. PMID 19370775.
- ↑ "Nitazoxanide for chronic hepatitis C". The Cochrane Database of Systematic Reviews (4): CD009182. April 2014. doi:10.1002/14651858.CD009182.pub2. PMID 24706397.
- ↑ "Nitazoxanide". MedlinePlus. https://www.nlm.nih.gov/medlineplus/druginfo/meds/a603017.html.
- ↑ 23.0 23.1 "Update on Nitazoxanide: A Multifunctional Chemotherapeutic Agent". Current Drug Discovery Technologies 15 (3): 201–213. 2018. doi:10.2174/1570163814666170727130003. PMID 28748751.
- ↑ "Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level". The Journal of Biological Chemistry 284 (43): 29798–29808. October 2009. doi:10.1074/jbc.M109.029470. PMID 19638339.
- ↑ "Nitazoxanide: an important advance in anti-parasitic therapy". The American Journal of Tropical Medicine and Hygiene 68 (4): 382–383. April 2003. doi:10.4269/ajtmh.2003.68.382. PMID 12875283.
- ↑ "Clinical Implications of Antiviral Resistance in Influenza". Viruses 7 (9): 4929–4944. September 2015. doi:10.3390/v7092850. PMID 26389935. "Oral nitazoxanide is an available, approved antiparasitic agent (e.g., against cryptosporidium, giardia) with established safety profiles. Recently, it has been shown (together with its active metabolite tizoxanide) to possess anti-influenza activity by blocking haemagglutinin maturation/trafficking, and acting as an interferon-inducer [97]. ... A large, multicenter, Phase 3 randomized-controlled trial comparing nitazoxanide, oseltamivir, and their combination in uncomplicated influenza is currently underway (NCT01610245).".
Figure 1: Molecular targets and potential antiviral treatments against influenza virus infection - ↑ "Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19". Journal of Virus Eradication 6 (2): 52–60. April 2020. doi:10.1016/S2055-6640(20)30017-0. PMID 32405422.
Further reading
- "Parasitic infections". American Journal of Transplantation 4 (Suppl 10): 142–155. November 2004. doi:10.1111/j.1600-6135.2004.00677.x. PMID 15504227.
External links
- "Nitazoxanide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/nitazoxanide.
 |

