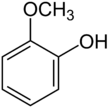
Chemistry:Guaiacol
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methoxyphenol | |||
| Other names
o-Methoxyphenol
O-Methylcatechol[2] 2-Hydroxyanisole Pyroguaiac acid Pyrocatechol monomethyl ether 1-hydroxy-2-methoxybenzene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H8O2 | |||
| Molar mass | 124.139 g·mol−1 | ||
| Appearance | colorless oil or crystalline solid | ||
| Density | 1.112 g/cm3, liquid 1.129 g/cm3, crystals | ||
| Melting point | 26–29 °C (79–84 °F; 299–302 K) | ||
| Boiling point | 204–206 °C (399–403 °F; 477–479 K) | ||
| 23.3 g/L at 25 °C | |||
| Related compounds | |||
Related methoxyphenols
|
Mequinol 3-Methoxyphenol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Guaiacol (/ˈɡwaɪəkɒl/) is an organic compound with the formula C6H4(OH)(OCH3). It is a phenolic compound containing a methoxy functional group. Guaiacol appears as a viscous colorless oil, although aged or impure samples are often yellowish. It occurs widely in nature and is a common product of the pyrolysis of wood.[3]
Occurrence
Guaiacol is usually derived from guaiacum or wood creosote.
It is produced by a variety of plants.[4] It is also found in essential oils from celery seeds, tobacco leaves, orange leaves, and lemon peels.[5] The pure substance is colorless, but samples become yellow upon exposure to air and light. The compound is present in wood smoke, resulting from the pyrolysis of lignin. The compound contributes to the flavor of many substances such as whiskey[6] and roasted coffee.[7]
Preparation
The compound was first isolated by Otto Unverdorben in 1826.[8] Guaiacol is produced by methylation of o-catechol, for example using potash and dimethyl sulfate:[3]
- C6H4(OH)2 + (CH3O)2SO2 → C6H4(OH)(OCH3) + HO(CH3O)SO2
Laboratory methods
Guaiacol can be prepared by diverse routes in the laboratory. o-Anisidine, derived in two steps from anisole, can be hydrolyzed via its diazonium derivative. Guaiacol can be synthesized by the dimethylation of catechol followed by selective mono-demethylation.[9]
- C6H4(OCH3)2 + C2H5SNa[10] → C6H4(OCH3)(ONa) + C2H5SCH3
Uses and chemical reactions
Syringyl/guaiacyl ratio
Lignin, comprising a major fraction of biomass, is sometimes classified according to the guaiacyl component. Pyrolysis of lignin from gymnosperms gives more guaiacol, resulting from removal of the propenyl group of coniferyl alcohol. These lignins are said to have a high guaiacyl (or G) content. In contrast, lignins derived from sinapyl alcohol affords syringol. A high syringyl (or S) content is indicative of lignin from angiosperms.[11] Sugarcane bagasse is one useful source of guaiacol; pyrolysis of the bagasse lignins yields compounds including guaiacol, 4-methylguaiacol and 4-vinylguaiacol.[12]
Chemical intermediate
Guaiacol is a useful precursor for the synthesis of other compounds.[13] Being derived from biomass, it is a potential component or precursor to "green fuels".[14]
Guaiacol is also a useful reagent for the quantification of peroxidases, as in the presence of hydrogen peroxide these enzymes will catalyse with it the formation of tetraguaiacol,[15] a coloured compound that can be quantified by its absorbance at 420–470 nm, following the equation:
4 guaiacol (colourless) + 2 H2O2 → tetraguaiacol (coloured) + 8 H2O.
Medicinal and food
Guaiacol is a precursor to various flavorants, such as eugenol.[16] An estimated 85% of the world's supply of vanillin comes from guaiacol. Because consumers tend to prefer natural vanillin to synthetic vanillin, methods such as microbial fermentation have been adopted. The route entails the condensation reaction of glyoxylic acid with guaiacol to give mandelic acid, which is oxidized to produce phenylglyoxylic acid. This acid undergoes a decarboxylation to afford vanillin.[17] The crude vanillin product can then be purified with vacuum distillation and recrystallization.
Guaiacol is also used medicinally as an expectorant, antiseptic, and local anesthetic.[18]
Guaiacol is produced in the gut of desert locusts, Schistocerca gregaria, by the breakdown of plant material. This process is undertaken by the gut bacterium Pantoea agglomerans (Enterobacter). It is one of the main components of the pheromones that cause locust swarming.[19]
Safety
Methoxyphenols are potential biomarkers of biomass smoke exposure, such as from inhalation of woodsmoke. Dietary sources of methoxyphenols overwhelm the contribution from inhalational exposures to woodsmoke.[20]
See also
References
- ↑ Merck Index (13th ed.). p. 4568.
- ↑ "List of synonyms for guaiacol". http://www.chemindustry.com/chemicals/464173.html.
- ↑ 3.0 3.1 Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef et al.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ↑ Duffey, S. S.; Aldrich, J. R.; Blum, M. S. (1977). "Biosynthesis of phenol and guaiacol by the hemipteran Leptoglossus phyllopus". Comparative Biochemistry and Physiology B 56 (2B): 101–102. doi:10.1016/0305-0491(77)90029-3. PMID 830476.
- ↑ Burdock, G. A. (1995). Encyclopedia of Food and Color Additives. Boca Raton, FL: CRC Press. pp. 1244–1245. ISBN 978-0849394126.
- ↑ Gallegos, Jenna (August 17, 2017). "The best way to drink whiskey, according to science". The Washington Post. https://www.washingtonpost.com/news/speaking-of-science/wp/2017/08/17/the-best-way-to-drink-whiskey-according-to-science/. "Guaiacol is what gives whiskey that smoky, spicy, peaty flavor."
- ↑ Dorfner, R.; Ferge, T.; Kettrup, A.; Zimmermann, R.; Yeretzian, C. (Sep 2003). "Real-time monitoring of 4-vinylguaiacol, guaiacol, and phenol during coffee roasting by resonant laser ionization time-of-flight mass spectrometry". Journal of Agricultural and Food Chemistry 51 (19): 5768–5773. doi:10.1021/jf0341767. ISSN 0021-8561. PMID 12952431.
- ↑ Stevens, M. E.; Ronan, A. K.; Sourkes, T. S.; E. M., Boyd (1943). "On the Expectorant Action of Creosote and the Guaiacols". Canadian Medical Association Journal 48 (2): 124–127. PMID 20322688.
- ↑ Mirrington, R. N.; Feutrill, G. I. (1988). "Orcinol Monomethyl Ether". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv6p0859.; Collective Volume, 6, pp. 859
- ↑ Sodium ethanethiolate
- ↑ Li, Laigeng; Cheng, Xiao Fei; Leshkevich, Jacqueline; Umezawa, Toshiaki; Harding, Scott A.; Chiang, Vincent L. (2001). "The Last Step of Syringyl Monolignol Biosynthesis in Angiosperms is Regulated by a Novel Gene Encoding Sinapyl Alcohol Dehydrogenase". The Plant Cell 13 (7): 1567–1586. doi:10.1105/tpc.010111. PMID 11449052.
- ↑ del Río, José C.; Lino, Alessandro G.; Colodette, Jorge L.; Lima, Claudio F.; Gutiérrez, Ana; Martínez, Ángel T.; Lu, Fachuang; Ralph, John et al. (2015-10-01). "Differences in the chemical structure of the lignins from sugarcane bagasse and straw" (in en). Biomass and Bioenergy 81: 322–338. doi:10.1016/j.biombioe.2015.07.006. ISSN 0961-9534.
- ↑ Liao, Chun-Chen (2005). "Masked o-benzoquinone strategy in organic synthesis: Short and efficient construction of cis-decalins and linear triquinanes from 2-methoxyphenols". Pure and Applied Chemistry 77 (7): 1221–1234. doi:10.1351/pac200577071221.
- ↑ Saidi, Majid; Samimi, Fereshteh; Karimipourfard, Dornaz; Nimmanwudipong, Tarit; Gates, Bruce C.; Rahimpour, Mohammad Reza (2014). "Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation". Energy Environ. Sci. 7: 103–129. doi:10.1039/C3EE43081B.
- ↑ "Oxidation of Guaiacol by Lignin Peroxidase. Role of veratryl alcohol". Journal of Biological Chemistry 270 (38): 22254–8. 1995. doi:10.1074/jbc.270.38.22254. PMID 7673205.
- ↑ Allen, C. F. H.; Gates, J. W., Jr (1955). "o-Eugenol". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv3P0418.; Collective Volume, 3, pp. 418
- ↑ Esposito, Lawrence J.; Formanek, K.; Kientz, G.; Mauger, F.; Maureaux, V.; Robert, G.; Truchet, F. (1997). "Vanillin". Kirk–Othmer Encyclopedia of Chemical Technology. 24 (4th ed.). New York, NY: John Wiley & Sons. pp. 812–825.
- ↑ "Guaiacol". 2019-11-02. https://www.drugbank.ca/drugs/DB11359.
- ↑ Dillon, Rod J.; Vennard, Chris T.; Charnley, A. Keith (2000-02-24). "Pheromones: Exploitation of gut bacteria in the locust". Nature 403 (6772): 851. doi:10.1038/35002669. PMID 10706273. Bibcode: 2000Natur.403..851D.
- ↑ Smith, K. R. (2005). "Critical review of the health effects of woodsmoke". School of Public Health, University of Berkeley. http://ehs.sph.berkeley.edu/krsmith/publications/2005%20pubs/HC%20woodsmoke%20report%20Mar%2031%2005%20%28rev%29.pdf.
 |