Chemistry:Palladium(II) bis(acetylacetonate)
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Palladium(II) 2,4-pentanedionate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C10H14O4Pd | |
| Molar mass | 304.64 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.79 g/cm3 |
| Melting point | 200 to 251 °C (392 to 484 °F; 473 to 524 K) (decomposes) |
| Structure[2] | |
| monoclinic | |
| P21/n, No. 14 | |
a = 9.9119 Å, b = 5.2232 Å, c = 10.3877 Å α = 90°, β = 95.807°, γ = 90°
| |
Lattice volume (V)
|
535.04 Å3 |
Formula units (Z)
|
2 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319 | |
| P210, P240, P241, P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
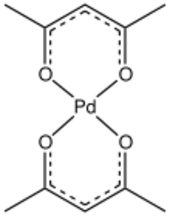
Palladium(II) bis(acetylacetonate) is a compound with formula Pd(C5H7O2)2. This yellow solid is the most common palladium complex of acetylacetonate. This compound is commercially available and used as a catalyst precursor in organic synthesis. The molecule is relatively planar with idealized D2h symmetry.[2]
See also
References
- ↑ Palladium(II) acetylacetonate at Sigma-Aldrich
- ↑ 2.0 2.1 Hamid, Mazhar; Zeller, Matthias; Hunter, Allen D.; Mazhar, Muhammad; Tahir, Asif Ali (2005). "Redetermination of bis(2,4-pentanedionato)palladium(II)". Acta Crystallographica Section E 61 (11): m2181–m2183. doi:10.1107/S1600536805030692.
- ↑ "4-Oxopent-2-en-2-olate;palladium(2+)" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/3035388#section=Safety-and-Hazards.
 |

