Chemistry:Platinum(II) bis(acetylacetonate)
From HandWiki
| |
| |

| |
| Names | |
|---|---|
| Other names
Bis(2,4-pentanedionato)platinum; Platinum bis(acetylacetonate); Bis(acetylacetonato)platinum
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C10H14O4Pt | |
| Molar mass | 393.302 g·mol−1 |
| Appearance | yellow solid |
| Density | 2.362 g/cm3 |
| Melting point | 239.4 C (dec.)[1] |
| insoluble | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H312, H315, H319, H332, H335, H361 | |
| P201, P202, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P304+312, P304+340, P305+351+338, P308+313, P312, P321, P322, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
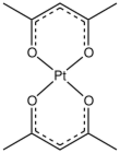
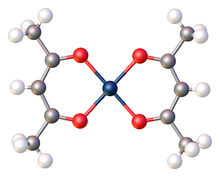
Platinum(II) bis(acetylacetonate) is the coordination compound with the formula Pt(O2C5H7)2, abbreviated Pt(acac)2. The homoleptic acetylacetonate complex of platinum(II), it is a yellow, benzene-soluble solid. According to X-ray crystallography, the Pt center is square planar.[2] The compound is a widely used precursor to platinum-based catalysts.Cite error: Closing </ref> missing for <ref> tag
See also
References
- ↑ Flanagan, Scott; Hall, Eric; Bowie, Wade; Fuhs, James W.; Logan, Robbie; Maniei, Farzaneh; Hunt, Andrew (2005). "A design-of-experiments approach to modeling activity coefficients in solvent mixtures: a case study using platinum(ii) acetylacetonate in mixtures of acetone, cyclohexanol, 1,2,3,4-tetrahydronaphthalene and propylene carbonate". Green Chemistry 7 (5): 333. doi:10.1039/b418130a. ISSN 1463-9262.
- ↑ Ha, Kwang (2011). "Crystal Structure of Bis(pentane-2,4-dionato)κ2O,O')platinum(II), Pt(C5H7O2)2". Zeitschrift für Kristallographie - New Crystal Structures 226 (3). doi:10.1524/ncrs.2011.0147.
 |

