Chemistry:Permanganate
| |
| |
| Names | |
|---|---|
| Systematic IUPAC name
Permanganate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MnO−4 | |
| Molar mass | 118.934 g·mol−1 |
| Conjugate acid | Permanganic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
A permanganate (/pərˈmæŋɡəneɪt, pɜːr-/)[1] is a chemical compound with the manganate(VII) ion, MnO−4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure.[2] Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).[3]
- 8MnO−4 + 3C2H3Cl3 → 6CO2 + 8MnO2 + H+ + 4H2O + 9Cl−
In an acidic solution, permanganate(VII) is reduced to the pale pink manganese(II) (Mn2+) with an oxidation state of +2.
- 8 H+ + MnO−4 + 5 e− → Mn2+ + 4 H2O
In a strongly basic or alkaline solution, permanganate(VII) is reduced to the green manganate ion, MnO2−4 with an oxidation state of +6.
- MnO−4 + e− → MnO2−4
In a neutral solution, however, it gets reduced to the brown manganese dioxide MnO2 with an oxidation state of +4.
- 2 H2O + MnO−4 + 3 e− → MnO2 + 4 OH−
Production
Permanganates can be produced by oxidation of manganese compounds such as manganese chloride or manganese sulfate by strong oxidizing agents, for instance, sodium hypochlorite or lead dioxide:
- 2 MnCl2 + 5 NaClO + 6 NaOH → 2 NaMnO4 + 9 NaCl + 3 H2O
- 2 MnSO4 + 5 PbO2 + 3 H2SO4 → 2 HMnO4 + 5 PbSO4 + 2 H2O
It may also be produced by the disproportionation of manganates, with manganese dioxide as a side-product:
- 3 Na2MnO4 + 2 H2O → 2 NaMnO4 + MnO2 + 4 NaOH
They are produced commercially by electrolysis or air oxidation of alkaline solutions of manganate salts (MnO2−4).[4]

Usage
This is a common and strong disinfectant, used regularly to sanitize baths, toilets, and wash basins[citation needed]. It is a cheap and extremely effective compound for the task.[citation needed]
Properties
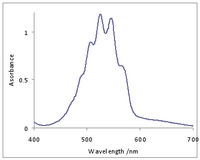
Permanganates(VII) are salts of permanganic acid. They have a deep purple colour, due to a charge transfer transition from oxo ligand p orbitals to empty orbitals derived from manganese(VII) d orbitals.[5] Permanganate(VII) is a strong oxidizer, and similar to perchlorate. It is therefore in common use in qualitative analysis that involves redox reactions (permanganometry). According to theory, permanganate is strong enough to oxidize water, but this does not actually happen to any extent. Besides this, it is stable.
It is a useful reagent, but it is not very selective with organic compounds. Potassium permanganate is used as a disinfectant and water treatment additive in aquaculture.[6]
Manganates(VII) are not very stable thermally. For instance, potassium permanganate decomposes at 230 °C to potassium manganate and manganese dioxide, releasing oxygen gas:
- 2 KMnO4 → K2MnO4 + MnO2 + O2
A permanganate can oxidize an amine to a nitro compound,[7][8] an alcohol to a ketone,[9] an aldehyde to a carboxylic acid,[10][11] a terminal alkene to a carboxylic acid,[12] oxalic acid to carbon dioxide,[13] and an alkene to a diol.[14] This list is not exhaustive.
In alkene oxidations one intermediate is a cyclic Mn(V) species:[15]
Compounds
- Ammonium permanganate, NH4MnO4
- Barium permanganate, Ba(MnO4)2
- Calcium permanganate, Ca(MnO4)2
- Potassium permanganate, KMnO4
- Sodium permanganate, NaMnO4
- Silver permanganate, AgMnO4
Safety
The fatal dose of permanganate is about 10 g, and several fatal intoxications have occurred. The strong oxidative effect leads to necrosis of the mucous membrane. For example, the esophagus is affected if the permanganate is swallowed. Only a limited amount is absorbed by the intestines, but this small amount shows severe effects on the kidneys and on the liver.[16][17]
See also
- Perchlorate, a similar ion with a chlorine(VII) center
- Permanganate index
- Chromate, which is isoelectronic with permanganate
- Pertechnetate
References
- ↑ "Permanganate". Merriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/permanganate.
- ↑ Sukalyan Dash, Sabita Patel; Bijay K. Mishra (2009). "Oxidation by permanganate: synthetic and mechanistic aspects". Tetrahedron 65 (4): 707–739. doi:10.1016/j.tet.2008.10.038.
- ↑ "Geo-Cleanse International, INC. | Permanganate". http://geocleanse.com/permanaganate.asp.
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Carlos A. Murillo; Manfred Bochmann (1999). Advanced Inorganic Chemistry (6th ed.). New York: John Wiley & Sons, Inc.. p. 770. ISBN 978-0471199571.
- ↑ Miessler, Gary L.; Fischer, Paul J.; Tarr, Donald A. (2014). Inorganic Chemistry (5th ed.). Pearson. p. 430. ISBN 978-0321811059.
- ↑ Syndel. "Potassium Permanganate Sodium Disinfectant". https://syndel.com/product/potassium-permanganate/.
- ↑ A. Calder, A. R. Forrester1, and S. P. Hepburn (1972). "2-methyl-2-nitrosopropane and its dimer". Organic Syntheses 6: 803. http://www.orgsyn.org/demo.aspx?prep=CV6P0803.; Collective Volume, 52, pp. 77
- ↑ Nathan Kornblum and Willard J. Jones (1963). "4-nitro-2,2,4-trimethylpentane". Organic Syntheses 5: 845. http://www.orgsyn.org/demo.aspx?prep=CV5P0845.; Collective Volume, 43, pp. 87
- ↑ J. W. Cornforth (1951). "Ethyl pyruvate". Organic Syntheses 4: 467. http://www.orgsyn.org/demo.aspx?prep=CV4P0467.; Collective Volume, 31, pp. 59
- ↑ R. L. Shriner and E. C. Kleiderer (1930). "Piperonylic acid". Organic Syntheses 2: 538. http://www.orgsyn.org/demo.aspx?prep=CV2P0538.; Collective Volume, 10, pp. 82
- ↑ John R. Ruhoff (1936). "n-heptanoic acid". Organic Syntheses 2: 315. http://www.orgsyn.org/demo.aspx?prep=CV2P0315.; Collective Volume, 16, pp. 39
- ↑ Donald G. Lee, Shannon E. Lamb, and Victor S. Chang (1981). "Carboxylic acids from the oxidation of terminal alkenes by permanganate: nonadecanoic acid". Organic Syntheses 7: 397. http://www.orgsyn.org/demo.aspx?prep=CV7P0397.; Collective Volume, 60, pp. 11
- ↑ "Revising the Mechanism of the Permanganate/Oxalate Reaction". J. Phys. Chem. A 108 (50): 11026. 2004. doi:10.1021/jp047061u. Bibcode: 2004JPCA..10811026K.
- ↑ E. J. Witzemann, Wm. Lloyd Evans, Henry Hass, and E. F. Schroeder (1931). "dl-glyceraldehyde ethyl acetal". Organic Syntheses 2: 307. http://www.orgsyn.org/demo.aspx?prep=CV2P0307.; Collective Volume, 11, pp. 52
- ↑ Lee, Donald G.; Chen, Tao (1993), "Reduction of manganate(VI) by mandelic acid and its significance for development of a general mechanism of oxidation of organic compounds by high-valent transition metal oxides", J. Am. Chem. Soc. 115 (24): 11231–36, doi:10.1021/ja00077a023.
- ↑ Ong, K. L.; Tan, T. H.; Cheung, W. L. (1997). "Potassium permanganate poisoning – a rare cause of fatal self poisoning". Emergency Medicine Journal 14 (1): 43–45. doi:10.1136/emj.14.1.43. PMID 9023625.
- ↑ Young, R.; Critchley, J. A.; Young, K. K.; Freebairn, R. C.; Reynolds, A. P.; Lolin, Y. I. (1996). "Fatal acute hepatorenal failure following potassium permanganate ingestion". Human & Experimental Toxicology 15 (3): 259–61. doi:10.1177/096032719601500313. PMID 8839216.
 |

