Chemistry:Silver permanganate
From HandWiki
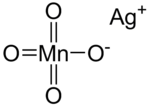
| |
| Names | |
|---|---|
| IUPAC name
Silver(I) permanganate
| |
| Systematic IUPAC name
Silver(I) manganate(VII) | |
| Other names
Argentous permanganate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| AgMnO4 | |
| Molar mass | 226.804 g/mol |
| Appearance | purple crystals or gray powder |
| Density | 4.27 g/cm3 |
| Melting point | 160 °C (320 °F; 433 K) (decomposes) |
| 0.55 g/100 mL (0 °C) 1.69 g/100 mL (30 °C) | |
| −63.0·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| Hazards | |
| Main hazards | Eye irritant |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H272, H312, H319, H332 | |
| P210, P220, P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P311, P321, P330, P337+313, P362+364Script error: No such module "Preview warning".Category:GHS errors, P370+378, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Silver permanganate is an inorganic compound with the chemical formula AgMnO4. This salt is a purple crystal adopting a monoclinic crystal system.[1] It decomposes when heated or mixed with water, and heating to high temperature may lead to explosion. The compound is used in gas masks.[citation needed]
Production
It can be produced through the reaction of silver nitrate and potassium permanganate:[2]
- AgNO3 + KMnO4 → AgMnO4 + KNO3
References
- ↑ Boonstra, E. G. (14 August 1968). "The crystal structure of silver permanganate". Acta Crystallographica Section B 24 (8): 1053–1062. doi:10.1107/S0567740868003699. Bibcode: 1968AcCrB..24.1053B.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
 |
