Chemistry:Phosphoribosyl pyrophosphate
| |
| Names | |
|---|---|
| IUPAC name
α-D-Ribofuranose 1′-(trihydrogen diphosphate) 5′-(dihydrogen phosphate)
| |
| Systematic IUPAC name
(2R,3R,4S,5R)-3,4-Dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl trihydrogen diphosphate | |
| Other names
5-phospho-α-D-ribose 1-diphosphate
PRPP | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| MeSH | Phosphoribosyl+pyrophosphate |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H13O14P3 | |
| Molar mass | 390.07 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
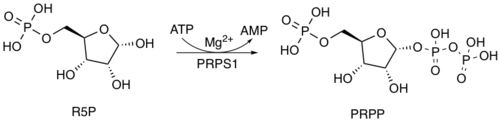
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA.[1][2][3] The vitamins thiamine[4] and cobalamin,[5] and the amino acid tryptophan also contain fragments derived from PRPP.[6] It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase:[7]
It plays a role in transferring phospho-ribose groups in several reactions, some of which are salvage pathways:[8]
| Enzyme | Reactant | Product |
|---|---|---|
| adenine phosphoribosyltransferase | adenine | AMP[9] |
| hypoxanthine-guanine phosphoribosyltransferase | guanine | GMP[10] |
| hypoxanthine-guanine phosphoribosyltransferase | hypoxanthine | IMP[10] |
| nicotinate phosphoribosyltransferase | nicotinate | nicotinate riboside[11] |
| orotate phosphoribosyltransferase | orotate | OMP[12] |
| uracil phosphoribosyltransferase | uracil | UMP[13] |
| xanthine phosphoribosyltransferase | xanthine | XMP[14] |
In de novo generation of purines, the enzyme amidophosphoribosyltransferase acts upon PRPP to create phosphoribosylamine.[2] The histidine biosynthesis pathway involves the reaction between PRPP and ATP, which activates the latter to ring cleavage. Carbon atoms from ribose in PRPP form the linear chain and part of the imidazole ring in histidine.[15][16][17] The same is true for the biosynthesis of tryptophan, with the first step being N-alkylation of anthranilic acid catalysed by the enzyme anthranilate phosphoribosyltransferase.[15][18][19]
Increased PRPP
Increased levels of PRPP are characterized by the overproduction and accumulation of uric acid leading to hyperuricemia and hyperuricosuria. It is one of the causes of gout.[20]
Increased levels of PRPP are present in Lesch–Nyhan Syndrome. Decreased levels of hypoxanthine guanine phosphoribosyl transferase (HGPRT) causes this accumulation, as PRPP is a substrate used by HGPRT during purine salvage.[21]
See also
- 5-Aminoimidazole ribotide
- Purine biosynthesis
- Pyrimidine biosynthesis
References
- ↑ R. Caspi (2009-01-13). "Pathway: 5-aminoimidazole ribonucleotide biosynthesis I". MetaCyc Metabolic Pathway Database. https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-6121.
- ↑ 2.0 2.1 Zhang, Y.; Morar, M.; Ealick, S.E. (2008). "Structural biology of the purine biosynthetic pathway". Cellular and Molecular Life Sciences 65 (23): 3699–3724. doi:10.1007/s00018-008-8295-8. PMID 18712276.
- ↑ Gupta, Rani; Gupta, Namita (2021). "Nucleotide Biosynthesis and Regulation". Fundamentals of Bacterial Physiology and Metabolism. pp. 525–554. doi:10.1007/978-981-16-0723-3_19. ISBN 978-981-16-0722-6.
- ↑ Chatterjee, Abhishek; Hazra, Amrita B.; Abdelwahed, Sameh; Hilmey, David G.; Begley, Tadhg P. (2010). "A "Radical Dance" in Thiamin Biosynthesis: Mechanistic Analysis of the Bacterial Hydroxymethylpyrimidine Phosphate Synthase". Angewandte Chemie International Edition 49 (46): 8653–8656. doi:10.1002/anie.201003419. PMID 20886485.
- ↑ R. Caspi (2019-09-23). "Pathway: 5-hydroxybenzimidazole biosynthesis (anaerobic)". MetaCyc Metabolic Pathway Database. https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-8097.
- ↑ Mehta, Angad P.; Abdelwahed, Sameh H.; Fenwick, Michael K.; Hazra, Amrita B.; Taga, Michiko E.; Zhang, Yang; Ealick, Steven E.; Begley, Tadhg P. (2015). "Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B12 Biosynthesis". Journal of the American Chemical Society 137 (33): 10444–10447. doi:10.1021/jacs.5b03576. PMID 26237670.
- ↑ Li, Sheng; Lu, Yongcheng; Peng, Baozhen; Ding, Jianping (January 2007). "Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site". Biochemical Journal 401 (1): 39–47. doi:10.1042/BJ20061066. PMID 16939420.
- ↑ R. Caspi (2022-02-15). "5-phospho-α-D-ribose 1-diphosphate". MetaCyc Metabolic Pathway Database. https://biocyc.org/compound?orgid=META&id=PRPP#tab=RXNS.
- ↑ Silva, Carlos H. T. P.; Silva, Marcio; Iulek, Jorge; Thiemann, Otavio H. (2008). "Structural Complexes of Human Adenine Phosphoribosyltransferase Reveal Novel Features of the APRT Catalytic Mechanism". Journal of Biomolecular Structure and Dynamics 25 (6): 589–597. doi:10.1080/07391102.2008.10507205. PMID 18399692.
- ↑ 10.0 10.1 Finette, Barry A.; Kendall, Heather; Vacek, Pamela M. (2002). "Mutational spectral analysis at the HPRT locus in healthy children". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 505 (1–2): 27–41. doi:10.1016/S0027-5107(02)00119-7. PMID 12175903.
- ↑ Vinitsky, A.; Grubmeyer, C. (1993). "A new paradigm for biochemical energy coupling. Salmonella typhimurium nicotinate phosphoribosyltransferase". Journal of Biological Chemistry 268 (34): 26004–26010. doi:10.1016/S0021-9258(19)74485-8. PMID 7503993.
- ↑ González-Segura, Lilian; Witte, John F.; McClard, Ronald W.; Hurley, Thomas D. (2007). "Ternary Complex Formation and Induced Asymmetry in Orotate Phosphoribosyltransferase". Biochemistry 46 (49): 14075–14086. doi:10.1021/bi701023z. PMID 18020427.
- ↑ Selwood, Trevor; Jaffe, Eileen K. (2012). "Dynamic dissociating homo-oligomers and the control of protein function". Archives of Biochemistry and Biophysics 519 (2): 131–143. doi:10.1016/j.abb.2011.11.020. PMID 22182754.
- ↑ Krenitsky, Thomas A.; Neil, Shannon M.; Miller, Richard L. (1970). "Guanine and Xanthine Phosphoribosyltransfer Activities of Lactobacillus casei and Escherichia coli". Journal of Biological Chemistry 245 (10): 2605–2611. doi:10.1016/S0021-9258(18)63113-8.
- ↑ 15.0 15.1 Voet, Donald (2016). Fundamentals of biochemistry : life at the molecular level. Judith G. Voet, Charlotte W. Pratt (Fifth ed.). Hoboken, NJ. ISBN 978-1-118-91840-1. OCLC 910538334. https://www.worldcat.org/oclc/910538334.
- ↑ R. Caspi (2008-10-10). "Pathway: L-histidine biosynthesis". MetaCyc Metabolic Pathway Database. https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=HISTSYN-PWY.
- ↑ Stepansky, A.; Leustek, T. (2006). "Histidine biosynthesis in plants". Amino Acids 30 (2): 127–142. doi:10.1007/s00726-005-0247-0. PMID 16547652.
- ↑ C.A. Fulcher (2010-02-12). "Pathway: L-tryptophan biosynthesis". MetaCyc Metabolic Pathway Database. https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=TRPSYN-PWY.
- ↑ Crawford, Irving P. (1989). "Evolution of a Biosynthetic Pathway: The Tryptophan Paradigm". Annual Review of Microbiology 43: 567–600. doi:10.1146/annurev.mi.43.100189.003031. PMID 2679363.
- ↑ Elliott, Katherine; Fitzsimons, David W. (16 September 2009). Purine and Pyrimidine Metabolism. John Wiley & Sons. pp. 143–158. ISBN 9780470717981.
- ↑ Cakmakli, Hasan F.; Torres, Rosa J.; Menendez, Araceli; Yalcin-Cakmakli, Gul; Porter, Christopher C.; Puig, Juan Garcia; Jinnah, H.A. (2019). "Macrocytic anemia in Lesch–Nyhan disease and its variants". Genetics in Medicine 21 (2): 353–360. doi:10.1038/s41436-018-0053-1. PMID 29875418.
 |