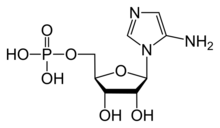
Chemistry:5-Aminoimidazole ribotide
| |
| Names | |
|---|---|
| IUPAC name
1-(5-Amino-1H-imidazol-1-yl)-1-deoxy-β-D-ribofuranose 5-(dihydrogen phosphate)
| |
| Systematic IUPAC name
[(2R,3S,4R,5R)-5-(5-Amino-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate | |
| Other names
AIR,
[5-(5-amino-1-imidazolyl)-3,4-dihydroxy-2-tetrahydrofuranyl]methyl dihydrogen phosphate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | aminoimidazole+ribotide |
PubChem CID
|
|
| |
| |
| Properties | |
| C8H14N3O7P | |
| Molar mass | 295.186 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5′-Phosphoribosyl-5-aminoimidazole (or aminoimidazole ribotide, AIR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA.[1] The vitamins thiamine[2][3] and cobalamin[4] also contain fragments derived from AIR.[5] It is an intermediate in the adenine pathway and is synthesized from 5′-phosphoribosylformylglycinamidine by AIR synthetase.[6]
Chemistry
5-aminoimidazole derivatives were considered unstable and therefore difficult to synthesize. The first non-enzymatic synthesis of 5-aminoimidazole ribotide (AIR) was only published in 1988[7] and general methodology for other examples was developed in the 1990s.[8][9]
Biosynthesis
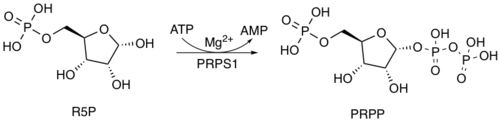
The furanose (5-carbon) sugar in AIR comes from the pentose phosphate pathway, which converts glucose (as its 6-phosphate derivative) into ribose 5-phosphate (R5P).[10] The subsequent reactions which attach the aminoimidazole portion of the molecule begin when R5P is activated as its pyrophosphate derivative, phosphoribosyl pyrophosphate (PRPP). This reaction is catalysed by ribose-phosphate diphosphokinase.[11]
Five biosynthetic steps complete the transformation.[1][12] The first enzyme, amidophosphoribosyltransferase, attaches ammonia from glutamine to the ribotide at its anomeric carbon, forming phosphoribosylamine (PRA):
- PRPP + glutamine → PRA + glutamate + PPi
Next, PRA is converted to glycineamide ribonucleotide (GAR) by the action of phosphoribosylamine—glycine ligase, forming an amide bond with glycine in a process driven by ATP:
- PRA + glycine + ATP → GAR + ADP + Pi
A third enzyme, phosphoribosylglycinamide formyltransferase, adds a formyl group from 10-formyltetrahydrofolate to GAR, giving phosphoribosyl-N-formylglycineamide (FGAR):
- GAR + 10-formyltetrahydrofolate → FGAR + tetrahydrofolate
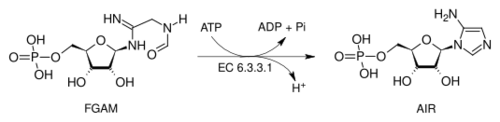
The penultimate step converts FGAR to an amidine by the action of phosphoribosylformylglycinamidine synthase, transferring an amino group from glutamine and giving 5′-phosphoribosylformylglycinamidine (FGAM) in a reaction that also requires ATP:
- FGAR + ATP + glutamine + H2O → FGAM + ADP + glutamate + Pi
FGAM is finally converted to AIR by the action of AIR synthetase which uses ATP to activate the terminal carbonyl group to attack by the nitrogen atom at the anomeric centre:
Use as an intermediate in biosynthesis
Purines
The purine ring system of the nucleotide inosine monophosphate is formed in a pathway from AIR[13] that begins when phosphoribosylaminoimidazole carboxylase converts it to the carboxylated derivative in the imidazole ring, 5′-phosphoribosyl-4-carboxy-5-aminoimidazole (CAIR).[14]
The same compound can be formed in a two-step pathway when the enzymes involved are 5-(carboxyamino)imidazole ribonucleotide synthase and 5-(carboxyamino)imidazole ribonucleotide mutase.[14]
Radical SAM reactions
Rearrangement reactions starting from AIR incorporate portions of the molecule into additional biochemical pathways. The enzymes involved are in the radical SAM superfamily of iron–sulfur proteins, which use S-adenosyl methionine as a cofactor to initiate the conversions via radical intermediates.[15][5]
Thiamine
The vitamin thiamine contains a pyrimidine ring system which is formed from AIR in a reaction catalysed by phosphomethylpyrimidine synthase.[2][16]
This reaction incorporates the blue, green and red fragments shown into the product, 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate.[3][17]
5-Hydroxybenzimidazole
In some anaerobes, AIR is a precursor to 5,6-dimethylbenzimidazole, which is incorporated into vitamin B12 in later steps of cobalamin biosynthesis.[5][18] The initial reaction is catalysed by 5-hydroxybenzimidazole synthase, EC 4.1.99.23, and forms 5-hydroxybenzimidazole:
All the carbon atoms of the product are transferred from AIR, as shown.[4][5]
References
- ↑ 1.0 1.1 R. Caspi (2009-01-13). "Pathway: inosine-5'-phosphate biosynthesis I". MetaCyc Metabolic Pathway Database. https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-6123.
- ↑ 2.0 2.1 R. Caspi (2011-09-14). "Pathway: superpathway of thiamine diphosphate biosynthesis I". MetaCyc Metabolic Pathway Database. https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=THISYN-PWY&detail-level=2#.
- ↑ 3.0 3.1 Chatterjee, Abhishek; Hazra, Amrita B.; Abdelwahed, Sameh; Hilmey, David G.; Begley, Tadhg P. (2010). "A "Radical Dance" in Thiamin Biosynthesis: Mechanistic Analysis of the Bacterial Hydroxymethylpyrimidine Phosphate Synthase". Angewandte Chemie International Edition 49 (46): 8653–8656. doi:10.1002/anie.201003419. PMID 20886485.
- ↑ 4.0 4.1 R. Caspi (2019-09-23). "Pathway: 5-hydroxybenzimidazole biosynthesis (anaerobic)". MetaCyc Metabolic Pathway Database. https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-8097.
- ↑ 5.0 5.1 5.2 5.3 Mehta, Angad P.; Abdelwahed, Sameh H.; Fenwick, Michael K.; Hazra, Amrita B.; Taga, Michiko E.; Zhang, Yang; Ealick, Steven E.; Begley, Tadhg P. (2015). "Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B12 Biosynthesis". Journal of the American Chemical Society 137 (33): 10444–10447. doi:10.1021/jacs.5b03576. PMID 26237670.
- ↑ Bhat, Balkrishen; Groziak, Michael P.; Leonard, Nelson J. (1990). "Nonenzymatic synthesis and properties of 5-aminoimidazole ribonucleotide (AIR). Synthesis of specifically 15N-labeled 5-aminoimidazole ribonucleoside (AIRs) derivatives". Journal of the American Chemical Society 112 (12): 4891–4897. doi:10.1021/ja00168a039.
- ↑ Groziak, M. P.; Bhat, B.; Leonard, N. J. (1988). "Nonenzymatic synthesis of 5-aminoimidazole ribonucleoside and recognition of its facile rearrangement". Proceedings of the National Academy of Sciences 85 (19): 7174–7176. doi:10.1073/pnas.85.19.7174. PMID 3174626. Bibcode: 1988PNAS...85.7174G.
- ↑ Al-Shaar, Adnan H. M.; Gilmour, David W.; Lythgoe, David J.; McClenaghan, Ian; Ramsden, Christopher A. (1992). "Preparation, structure and addition reactions of 4- and 5-aminoimidazoles". Journal of the Chemical Society, Perkin Transactions 1 (21): 2779–2788. doi:10.1039/P19920002779.
- ↑ Al-Shaar, Adnan H. M.; Chambers, Robert K.; Gilmour, David W.; Lythgoe, David J.; McClenaghan, Ian; Ramsden, Christopher A. (1992). "The synthesis of heterocycles via addition–elimination reactions of 4- and 5-aminoimidazoles". J. Chem. Soc., Perkin Trans. 1 (21): 2789–2811. doi:10.1039/P19920002789.
- ↑ Alfarouk, Khalid O.; Ahmed, Samrein B. M.; Elliott, Robert L.; Benoit, Amanda; Alqahtani, Saad S.; Ibrahim, Muntaser E.; Bashir, Adil H. H.; Alhoufie, Sari T. S. et al. (2020). "The Pentose Phosphate Pathway Dynamics in Cancer and Its Dependency on Intracellular pH" (in en). Metabolites 10 (7): 285. doi:10.3390/metabo10070285. PMID 32664469.
- ↑ Li, Sheng; Lu, Yongcheng; Peng, Baozhen; Ding, Jianping (January 2007). "Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site". Biochemical Journal 401 (1): 39–47. doi:10.1042/BJ20061066. PMID 16939420.
- ↑ Zhang, Y.; Morar, M.; Ealick, S.E. (2008). "Structural biology of the purine biosynthetic pathway". Cellular and Molecular Life Sciences 65 (23): 3699–3724. doi:10.1007/s00018-008-8295-8. PMID 18712276.
- ↑ Gupta, Rani; Gupta, Namita (2021). "Nucleotide Biosynthesis and Regulation". Fundamentals of Bacterial Physiology and Metabolism. pp. 525–554. doi:10.1007/978-981-16-0723-3_19. ISBN 978-981-16-0722-6.
- ↑ 14.0 14.1 Mathews, Irimpan I.; Kappock, T. Joseph; Stubbe, JoAnne; Ealick, Steven E. (1999). "Crystal structure of Escherichia coli PurE, an unusual mutase in the purine biosynthetic pathway". Structure 7 (11): 1395–1406. doi:10.1016/S0969-2126(00)80029-5. PMID 10574791.
- ↑ Holliday, Gemma L.; Akiva, Eyal; Meng, Elaine C.; Brown, Shoshana D.; Calhoun, Sara; Pieper, Ursula; Sali, Andrej; Booker, Squire J. et al. (2018). "Atlas of the Radical SAM Superfamily: Divergent Evolution of Function Using a "Plug and Play" Domain". Radical SAM Enzymes. Methods in Enzymology. 606. pp. 1–71. doi:10.1016/bs.mie.2018.06.004. ISBN 9780128127940.
- ↑ Challand, Martin R.; Driesener, Rebecca C.; Roach, Peter L. (2011). "Radical S-adenosylmethionine enzymes: Mechanism, control and function". Natural Product Reports 28 (10): 1709–1710. doi:10.1039/C1NP00036E. PMID 21779595.
- ↑ Begley, Tadhg P. (2006). "Cofactor biosynthesis: An organic chemist's treasure trove". Natural Product Reports 23 (1): 15–18. doi:10.1039/b207131m. PMID 16453030.
- ↑ Sokolovskaya, Olga M.; Shelton, Amanda N.; Taga, Michiko E. (2020). "Sharing vitamins: Cobamides unveil microbial interactions". Science 369 (6499). doi:10.1126/science.aba0165. PMID 32631870.