Chemistry:Pyrinuron
From HandWiki
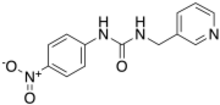
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-(4-Nitrophenyl)-N′-[(pyridin-3-yl)methyl]urea | |
| Other names
Pyriminil
Vacor | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H12N4O3 | |
| Molar mass | 272.264 g·mol−1 |
| Hazards | |
| Main hazards | Toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pyrinuron (Pyriminil, Vacor) is a chemical compound formerly used as a rodenticide.[1] Commercial distribution was voluntarily suspended in 1979 and it is not approved by the Environmental Protection Agency for use in the United States.[2] If it is ingested by humans in high doses, it may selectively destroy insulin-producing beta cells in the pancreas causing type 1 diabetes.[2] The neurodegeneration associated with Vacor is caused by its conversion to Vacor-mononucleotide (VMN) by NAMPT and VMN's subsequent activation of the NADase SARM1.[3]
References
- ↑ Vogel, R. P. (1982). "Poisoning with Vacor Rodenticide". Archives of Pathology and Laboratory Medicine 106 (3): 153. PMID 6895844.
- ↑ 2.0 2.1 "Pyriminil". U.S. National Library of Medicine. http://hazmap.nlm.nih.gov/category-details?id=6918&table=copytblagents.
- ↑ Loreto, Andrea; Angeletti, Carlo; Gu, Weixi; Osborne, Andrew; Nieuwenhuis, Bart; Gilley, Jonathan; Arthur-Farraj, Peter; Merlini, Elisa et al. (2021-06-23). "Potent activation of SARM1 by NMN analogue VMN underlies vacor neurotoxicity" (in en). bioRxiv: 2020.09.18.304261. doi:10.1101/2020.09.18.304261. https://www.biorxiv.org/content/10.1101/2020.09.18.304261v2.
 |
