Chemistry:Chloralose
| |
| Names | |
|---|---|
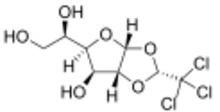
| IUPAC name
1,2-O-[(1R)-2,2,2-Trichloroethane-1,1-diyl]-α-D-glucofuranose
| |
| Systematic IUPAC name
(1R)-1-[(2R,3aR,4S,5R,6aR)-2-(Trichloromethyl)-tetrahydro-2H-furo[2,3-d][1,3]dioxol-5-yl]ethane-1,2-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| 85418 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | Chloralose |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C8H11Cl3O6 | |
| Molar mass | 309.52 g·mol−1 |
| Melting point | 176 to 182 °C (349 to 360 °F; 449 to 455 K) |
| Hazards | |
| Main hazards | Harmful if swallowed Harmful if inhaled |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H332, H336, H410 | |
| P261, P264, P270, P271, P273, P301+310, P304+312, P304+340, P312, P321, P330, P391, P403+233, P405, P501 | |
| Related compounds | |
Related compounds
|
Chloral hydrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chloralose (also known as α-chloralose) is an avicide, and a rodenticide used to kill mice in temperatures below 15 °C. It is also widely used in neuroscience and veterinary medicine as an anesthetic and sedative.[1] Either alone or in combination, such as with urethane, it is used for long-lasting, but light anesthesia.[2]
Chemically, it is a chlorinated acetal derivative of glucose.
It is listed in Annex I of Directive 67/548/EEC with the classification Harmful (Xn)
Chloralose exerts barbiturate-like actions on synaptic transmission in the brain, including potent effects at inhibitory γ-aminobutyric acid type A receptors (GABAAR).[3][4] A structural isomer of chloralose, β-chloralose (also called parachloralose in older literature), is inactive as a GABAAR modulator and also as a general anesthetic.[5]
Chloralose is often abused for its avicide properties. In the United Kingdom , protected birds of prey have been killed using the chemical. Legal use for bird control also often causes raptor mortalities from secondary poisoning, as well as primary poisoning of non-target species from eating bait, for example, kererū pigeon in New Zealand.[6]
References
- ↑ Silverman J, Muir WW (Jun 1993). "A review of laboratory animal anesthesia with chloral hydrate and chloralose". Lab Anim Sci. 43 (3): 210–6. PMID 8355479.
- ↑ Vogler, George A. (2006-01-01), Suckow, Mark A.; Weisbroth, Steven H.; Franklin, Craig L., eds., "Chapter 19 - Anesthesia and Analgesia" (in en), The Laboratory Rat (Second Edition), American College of Laboratory Animal Medicine (Burlington: Academic Press): pp. 627–664, ISBN 978-0-12-074903-4, https://www.sciencedirect.com/science/article/pii/B9780120749034500224, retrieved 2021-03-21
- ↑ R. A. Nicoll & J. M. Wojtowicz (1980). "The effects of pentobarbital and related compounds on frog motoneurons". Brain Research 191 (1): 225–237. doi:10.1016/0006-8993(80)90325-x. PMID 6247012.
- ↑ K. M. Garrett & J. Gan (1998). "Enhancement of gamma-aminobutyric acidA receptor activity by alpha-chloralose". The Journal of Pharmacology and Experimental Therapeutics 285 (2): 680–686. PMID 9580613.
- ↑ M. D. Krasowski & N. L. Harrison (2000). "The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations". British Journal of Pharmacology 129 (4): 731–743. doi:10.1038/sj.bjp.0703087. PMID 10683198.
- ↑ "Poisoned bird had enough toxin to 'kill a child'" (in en-GB). BBC News. 2020-07-26. https://www.bbc.com/news/uk-england-york-north-yorkshire-53543989.
 |
