Chemistry:Vitamin K antagonist
Vitamin K antagonists (VKA) are a group of substances that reduce blood clotting by reducing the action of vitamin K. The term "vitamin K antagonist" is technically a misnomer, as the drugs do not directly antagonize the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K. Vitamin K antagonists (VKAs) have been the mainstay of anticoagulation therapy for more than 50 years.
They are used as anticoagulant medications in the prevention of thrombosis, and in pest control, as rodenticides.
Mechanism of action
These drugs deplete the active form of the vitamin by inhibiting the enzyme vitamin K epoxide reductase and thus the recycling of the inactive vitamin K epoxide back to the active reduced form of vitamin K. The drugs are structurally similar to vitamin K and act as competitive inhibitors of the enzyme. The term "vitamin K antagonist" is a misnomer, as the drugs do not directly antagonise the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K.
Vitamin K is required for the proper production of certain proteins involved in the blood clotting process. For example, it is needed to carboxylate specific glutamic acid residues on prothrombin. Without these residues carboxylated, the protein will not form the appropriate conformation of thrombin, which is needed to produce the fibrin monomers that are polymerized to form clots.[1]
The action of this class of anticoagulants may be reversed by administering vitamin K for the duration of the anticoagulant's residence in the body, and the daily dose needed for reversal is the same for all drugs in the class. However, in the case of the second generation superwarfarins intended to kill warfarin-resistant rodents, the time of vitamin K administration may need to be prolonged to months, in order to combat the long residence time of the poison.[2]
The vitamin K antagonists can cause birth defects (teratogens).[3]
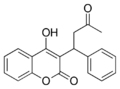
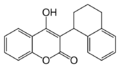
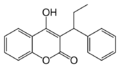
Coumarins (4-hydroxycoumarins)
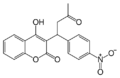
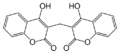
Coumarins (more accurately 4-hydroxycoumarins) are the most commonly used VKAs.
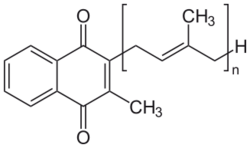
In medicine, the most commonly used VKA is warfarin.[4] Warfarin was initially used as a rodenticide, but made the transition to pharmaceutical. Eventually some rodents developed resistance to it. The "second generation" VKAs for dedicated use as rodenticides are sometimes called superwarfarins. These VKAs are enhanced to kill warfarin-resistant rodents. The enhancement to the molecule takes the form of a larger lipophilic group to enhance the fat solubility of the poison and greatly increase the time it acts within the animal's body.[5] However, as described above, the superwarfarins do not inhibit vitamin K and their effect is easily inhibited by vitamin K. Nevertheless, oral vitamin K may need to be given for times that may exceed a month (cases have been described needing as much as nine months vitamin K supplementation), in order to counter the effect of second-generation VKAs that have very long residence times in the fat of animals and humans.
For a more complete list of coumarins used as pharmaceuticals and rodenticides, see the main article on 4-hydroxycoumarins.
Warfarin (Coumadin)
Indandiones
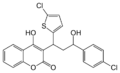
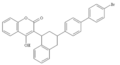
Another group of VKAs are 1,3-indandione derivatives. Pindone, chlorophacinone, and diphacinone are used as rodenticides. They are categorised as "first-generation" anticoagulants, and have similar effects as warfarin. They have been largely superseded by second-generation anticoagulants because warfarin-resistant rodents have become more common.[6]
Anisindione, fluindione, and phenindione are oral anticoagulant medicines with actions similar to warfarin. However, the indandiones are generally more toxic than warfarin, with hypersensitivity reactions involving many organs and sometimes resulting in death. They are therefore now rarely used.[7]
See also
References
- ↑ Suttie, J. W. (July 1980). "Mechanism Of Action Of Vitamin K: Synthesis Of Y-Carboxyglutamic Acid". Critical Reviews in Biochemistry 8 (2): 191–223. doi:10.3109/10409238009105469. PMID 6772376.
- ↑ Olmos V, López CM (2007). "Brodifacoum Poisoning with Toxicokinetic Data". Clinical Toxicology 45 (5): 487–9. doi:10.1080/15563650701354093. PMID 17503253.
- ↑ "Vitamin K antagonists and pregnancy outcome. A multi-centre prospective study". Thromb. Haemost. 95 (6): 949–57. June 2006. doi:10.1160/TH06-02-0108. PMID 16732373.
- ↑ "Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)". Chest 133 (6 Suppl): 160S–198S. June 2008. doi:10.1378/chest.08-0670. PMID 18574265. http://journal.publications.chestnet.org/article.aspx?articleid=1085927.
- ↑ Griminger P (July 1987). "Vitamin K antagonists: the first 50 years". J. Nutr. 117 (7): 1325–9. doi:10.1093/jn/117.7.1325. PMID 3302140. http://jn.nutrition.org/content/117/7/1325.full.pdf.
- ↑ The NRA Review of PINDONE (Report). National Registration Authority for Agricultural and Veterinary Chemicals, Australia. May 2002. Section 3.1.4. https://apvma.gov.au/sites/default/files/publication/14856-pindone-review-final-report.pdf. Retrieved 21 June 2017.
- ↑ Sean C Sweetman, ed (2009). Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. "Phenindione", p. 1369.
Further reading
- Published reviews, 2011-2016, regarding Vitamin K antagonists.
- Reynolds, Matthew R. (February 2013). "Discontinuation of Rivaroxaban: Filling in the Gaps" (editorial comment). J. Am. Coll. Cardiol. 61 (6): 659–60. doi:10.1016/j.jacc.2012.09.056. PMID 23391197. http://content.onlinejacc.org/article.aspx?articleID=1567631#tab1. Retrieved 5 April 2016. "The authors'... most likely explanation for the observed risk in the post-study transition period is not that rivaroxaban has some property resulting in a rebound effect, but rather that the high-risk... ROCKET AF trial [patients]... had a substantial difference in anticoagulation coverage during this period, and the event rates merely reflect the unmasking of their underlying risk... / Although this explanation is fairly persuasive, the evidence that the post-study excess stroke risk in rivaroxaban patients was the result of inadequate VKA [vitamin K antagonist] therapy remains somewhat circumstantial. The INRs were not collected as carefully during the post-trial period... [and] the authors do not provide any information on the use of bridging therapies... with unfractionated or low–molecular-weight heparin... [which] was not mandated by the study protocol during either temporary interruptions or at the end of the study. The bleeding rates reported in the current study also are counterintuitive: if the excess strokes in rivaroxaban patients were the result of underanticoagulation in the post-trial period, then why did these patients also have a higher bleeding risk? The investigators will need to scrutinize this large trial database further to understand these issues more fully.".
- Patel, Manesh R.; Anne S. Hellkamp; Yuliya Lokhnygina; Jonathan P. Piccini; Zhongxin Zhang; Surya Mohanty; Daniel E. Singer; Werner Hacke et al. (February 2013). "Outcomes of Discontinuing Rivaroxaban Compared With Warfarin in Patients With Nonvalvular Atrial Fibrillation: Analysis From the ROCKET AF Trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation)". J. Am. Coll. Cardiol. 61 (6): 651–658. doi:10.1016/j.jacc.2012.09.057. PMID 23391196. http://content.onlinejacc.org/article.aspx?articleID=1567632#tab1. Retrieved 5 April 2016. "We undertook a post-hoc analysis of data from the ROCKET AF (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation, n = 14,624) for stroke or non-CNS embolism within 30 days after temporary interruptions of 3 days or more, early permanent study drug discontinuation, and end-of-study transition to open-label therapy. / In atrial fibrillation patients who temporarily or permanently discontinued anticoagulation, the risk of stroke or non-CNS embolism was similar with rivaroxaban or warfarin. An increased risk of stroke and non-CNS embolism was observed in rivaroxaban-treated patients compared with warfarin-treated patients after the end of the study, underscoring the importance of therapeutic anticoagulation coverage during such a transition.".
- Agnelli, Giancarlo (2005). "Foreword: Contemporary issues in the management and treatment of atrial fibrillation" (supplement foreword). European Heart Journal Supplements 7 (Supplement C, 14 April): C3–C4. doi:10.1093/eurheartj/sui013. http://eurheartjsupp.oxfordjournals.org/content/ehjsupp/7/suppl_C/C3.full.pdf. Retrieved 5 April 2016. "This supplement represents the first publication of Thrombosis Quorum (TQ), a recently established international consortium of multidisciplinary thrombosis-related specialists dedicated to raising the priority of thrombosis. TQ aims to address the unmet clinical needs in the prevention and treatment of thromboembolic conditions, and promote the optimum management of patients with or at risk of these disorders by providing a cross-disciplinary forum for information exchange and debate. This [issue] incorporates a collection of state-of-the-art articles written by the TQ Steering Group and co-authors, and in this instance, focuses on the management and treatment of patients with atrial fibrillation (AF).". Note, because this issue foreword is over a decade old, its statements regarding limited available oral anticoagulants is no longer accurate.
 |