Chemistry:Ralmitaront
From HandWiki
Short description: Investigational antipsychotic drug
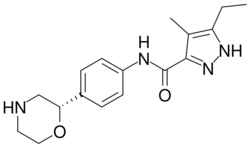 | |
| Clinical data | |
|---|---|
| Other names | RG-7906; RO-6889450 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H22N4O2 |
| Molar mass | 314.389 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ralmitaront (INN, USAN; developmental code names RG-7906 and RO-6889450) is an investigational antipsychotic drug which is undergoing a clinical trial for the treatment negative symptoms in schizophrenia and schizoaffective disorder.[1][2][3] Another clinical trial targeting acute psychotic symptoms of schizophrenia has been terminated due to lack of efficacy.[4] It is a partial agonist of the TAAR1.[5] The medication is being developed by the pharmaceutical company Hoffmann-La Roche.[1] Ralmitaront had completed phase 1 clinical trials.[1][6]
See also
References
- ↑ 1.0 1.1 1.2 "Ralmitaront - Roche". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800044887.
- ↑ "Beyond Dopamine Receptor Antagonism: New Targets for Schizophrenia Treatment and Prevention". International Journal of Molecular Sciences 22 (9): 4467. April 2021. doi:10.3390/ijms22094467. PMID 33922888.
- ↑ Clinical trial number NCT04512066 for "Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Effects of RO6889450 (Ralmitaront) in Patients With Schizophrenia or Schizoaffective Disorder and Negative Symptoms" at ClinicalTrials.gov
- ↑ Clinical trial number NCT03669640 for "A Trial of the Efficacy and the Safety of RO6889450 (Ralmitaront) vs Placebo in Patients With an Acute Exacerbation of Schizophrenia or Schizoaffective Disorder" at ClinicalTrials.gov
- ↑ "Ralmitaront: Relationships: Trace Amine-Associated Receptor 1". Global Substance Registration System (GSRS). U.S. Food and Drug Administration. https://gsrs.ncats.nih.gov/app/substance/QA8MW1Q80P.
- ↑ Clinical trial number NCT02699372 for "The Safety, Tolerability, Pharmacokinetics and Pharmacodynamics Study of RO6889450 in Healthy Volunteers" at ClinicalTrials.gov
 |

