Chemistry:Spiropentadiene
| |
| Names | |
|---|---|
| Preferred IUPAC name
Spiro[2.2]penta-1,4-diene | |
| Other names
Bowtiediene; Spiropenta-1,4-diene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C5H4 | |
| Molar mass | 64.087 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
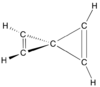
Spiropentadiene, or bowtiediene, is a hydrocarbon with formula C5H4. The simplest spiro-connected diene, it is very unstable—decomposing even below −100 °C—due to its high bond strain and does not occur in nature. Its synthesis was reported in 1991.[1][2]
Synthesis
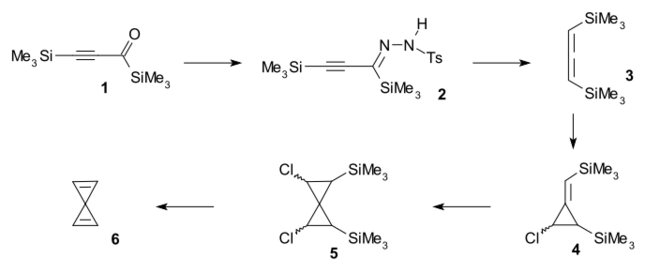
Spiropentadiene was synthesised from bistrimethylsilylpropynone 1 by reaction with p-toluenesulfonylhydrazide to tosylhydrazone 2 followed by treatment with sodium cyanoborohydride to allene 3 and followed by two successive reactions with chlorocarbene generated from methyllithium and dichloromethane to spiro compound 5. Spiropentadiene was trapped in a liquid nitrogen trap after reaction with TBAF in a double elimination reaction.
Derivatives
The derivative dichlorospiropentadiene has been reported.[3] An all-silicon derivative (Si5 frame, (tBuMe2Si)3Si side groups) is also known.[4] In contrast to the carbon parent this compound is stable with a melting point of 216 to 218 °C. The angle between the two rings as measured by X-ray single-crystal analysis is 78°.
References
- ↑ Billups, W. E.; Haley, M. M. (1991). "Spiropentadiene". Journal of the American Chemical Society 113 (13): 5084. doi:10.1021/ja00013a067.
- ↑ "Elusive bowtie pinned down - synthesis of spiropentadiene, a carbonaceous compound nicknamed bowtiediene because it is shaped like a bowtie". Science News. July 13, 1991. https://www.thefreelibrary.com/Elusive+bowtie+pinned+down.-a011134368.
- ↑ Saini, R.; Litosh, V.; Daniels, A.; Billups, W. (1999). "Synthesis and characterization of 1,4-dichlorospiropentadiene". Tetrahedron Letters 40 (34): 6157. doi:10.1016/S0040-4039(99)01293-9.
- ↑ Iwamoto, T.; Tamura, M.; Kabuto, C.; Kira, M. (2000). "A Stable Bicyclic Compound with Two Si=Si Double Bonds". Science 290 (5491): 504–506. doi:10.1126/science.290.5491.504. PMID 11039928. Bibcode: 2000Sci...290..504I.
 |