Chemistry:Tolimidone
From HandWiki
Short description: Chemical compound
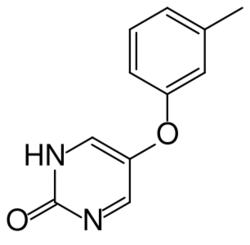 | |
| Clinical data | |
|---|---|
| Other names | CP-26154, MLR-1023 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H10N2O2 |
| Molar mass | 202.213 g·mol−1 |
| 3D model (JSmol) | |
| |
Tolimidone (CP-26154; MLR-1023) is a compound which was discovered by scientists at Pfizer, was found to stimulate secretion of gastric mucosa, and was in development by Pfizer as a drug candidate to treat gastric ulcers but was abandoned.[1][2][3][4] After the patent on the compound expired, scientists at the company Melior Discovery identified it as a potential drug candidate for diabetes through a phenotypic screen.[4] The company proceeded to show that MLR-1023 is an allosteric activator of Lyn kinase with an EC50 of 63 nM.[5][6] As of 2012 Melior was repurposing it for diabetes.[1][7] In June 2016, the company reported positive results from their Phase 2a clinical study in diabetic subjects[8][9]
References
- ↑ 1.0 1.1 "Chapter 9:Phenotypic In Vivo Screening to Identify New, Unpredicted Indications for Existing Drugs and Drug Candidates" (in en). Drug Repositioning: Bringing New Life to Shelved Assets and Existing Drugs. John Wiley & Sons. 2012. p. 270. ISBN 9781118274392. https://books.google.com/books?id=TqOBrx_xZgAC&pg=PA270.
- ↑ "Tolimidone" (in en). AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800023467.
- ↑ "Bronchodilator and antiulcer phenoxypyrimidinones". Journal of Medicinal Chemistry 23 (9): 1026–1031. September 1980. doi:10.1021/jm00183a012. PMID 7411545. "Compound 3 has been assigned the nonproprietary (USAN) name tolimidone".
- ↑ 4.0 4.1 "High throughput in vivo phenotypic screening for drug repurposing: Discovery of MLR-1023 a novel insulin sensitizer and novel Lyn kinase activator with clinical proof of concept". Bioorganic & Medicinal Chemistry 28 (9): 115425. May 2020. doi:10.1016/j.bmc.2020.115425. PMID 32201192.
- ↑ "The Lyn kinase activator MLR-1023 is a novel insulin receptor potentiator that elicits a rapid-onset and durable improvement in glucose homeostasis in animal models of type 2 diabetes". The Journal of Pharmacology and Experimental Therapeutics 342 (1): 23–32. July 2012. doi:10.1124/jpet.112.192187. PMID 22431203.[yes|permanent dead link|dead link}}]
- ↑ "MLR-1023 is a potent and selective allosteric activator of Lyn kinase in vitro that improves glucose tolerance in vivo". The Journal of Pharmacology and Experimental Therapeutics 342 (1): 15–22. July 2012. doi:10.1124/jpet.112.192096. PMID 22473614.
- ↑ "Melior Pharmaceuticals Announces Positive Results in Phase 2B Study with Tolimidone for Type 2 Diabetes". https://www.businesswire.com/news/home/20190514005095/en/Melior-Pharmaceuticals-Announces-Positive-Results-Phase-2B.
- ↑ "Melior Pharmaceuticals Announces Positive Phase 2A Results in Type 2 Diabetes Study". June 13, 2016. https://www.businesswire.com/news/home/20160613005028/en/Melior-Pharmaceuticals-Announces-Positive-Phase-2A-Results-in-Type-2-Diabetes-Study.
- ↑ "A novel non-PPARgamma insulin sensitizer: MLR-1023 clinicalproof-of-concept in type 2 diabetes mellitus". Journal of Diabetes and Its Complications 34 (5): 107555. May 2020. doi:10.1016/j.jdiacomp.2020.107555. PMID 32019723.
 |
